Selective Coupling
Reflecting work in the Suga & Vinogradov Labs
α,β-Dehydroalanine, ΔAla, is a highly reactive nonproteinogenic amino acid that serves as a versatile handle for modifying peptides, natural products, NPs, and proteins. While many ΔAla functionalization strategies exist, they often yield complex product mixtures, limiting their application. Published in the Journal of the American Chemical Society, researchers in the Hiroaki Suga Group at the University of Tokyo, in collaboration with the Vinogradov team at the National University of Singapore, present a highly selective Pd(II)-mediated coupling reaction that enables the precise modification of ΔAla-containing peptides and proteins.

Hiroaki Suga

Alexander Vinogradov
Key Findings
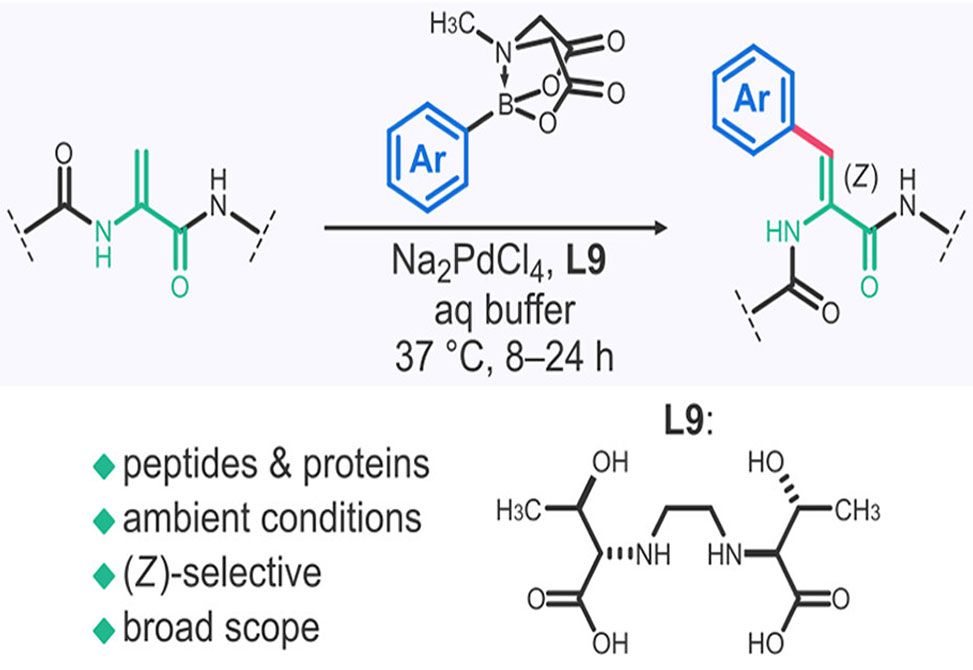
The reaction couples aryl N-methylimidodiacetic acid, MIDA, boronates to ΔAla-containing substrates, forming ΔzPhe derivatives with high regio- and stereoselectivity.
This transformation occurs in aqueous conditions at 37°C, within 24 hours, using fully unprotected peptides and proteins.
The use of N,N'-ethylene-bis-L-threonine, H2ebt, as a Pd(II) ligand is critical for accelerating the reaction while ensuring product purity.
The method is compatible with a broad range of peptides, NP-like compounds, and intact proteins, including ubiquitin and PstS.
A one-pot chemoribosomal synthesis strategy has been developed, integrating this chemistry with ribosomal peptide translation and enzymatic dehydration for ΔAla incorporation.
Applications
This method provides a powerful tool for late-stage peptide and protein modification, bioconjugation, and drug discovery. The ability to selectively derivatize ΔAla-containing natural products, such as thiopeptides, offers a route for tailoring bioactive molecules with enhanced pharmacological properties. Furthermore, its integration with in vitro translation technologies opens avenues for discovering bioactive peptide ligands through mRNA display screening.
This advancement represents a significant step forward in site-selective peptide functionalization, expanding the toolkit available for researchers working in chemical biology and peptide-based therapeutics.