Sequence Architecture
Reflecting work in the Woolfson Lab
The Woolfson Lab continues to break new ground in de novo protein design with the successful creation of mixed-charge A3B3 heterohexameric α-helical barrels, αHBs, assembled from distinct acidic, A, and basic, B, peptide chains. Drawing on well-established sequence-to-structure rules in coiled-coil assemblies, the team used programmed heptad repeats—particularly residues at the a, d, e, and g positions—to favor specific oligomeric states and prevent homomeric aggregation.
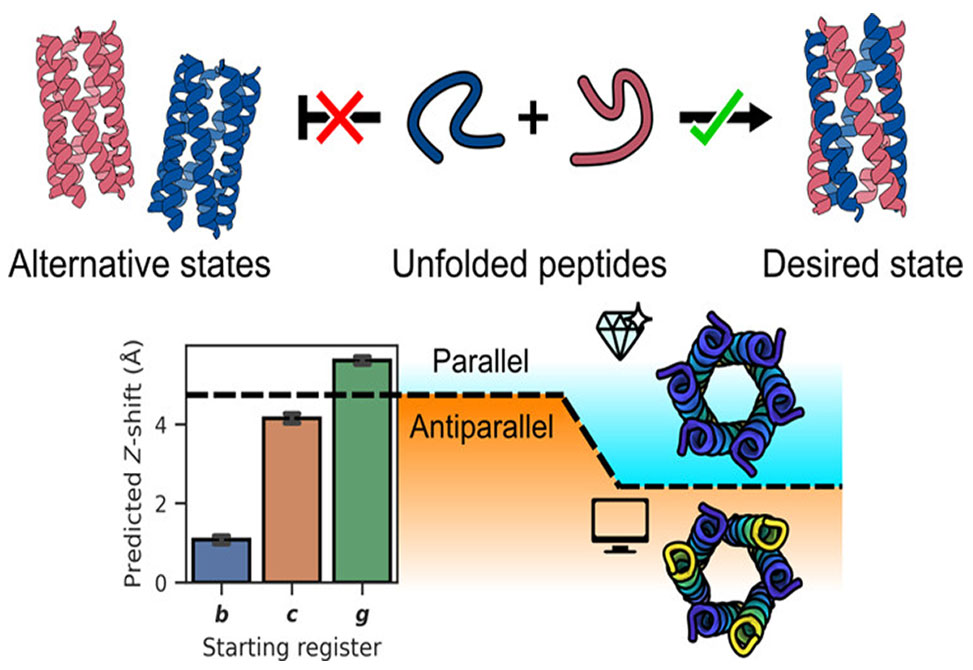
A key outcome of this work, which is published as an ASAP article in Biochemistry, is the elucidation of how subtle sequence architecture, namely the register, starting position, of the heptad repeat, can direct the relative orientation of helices within a multichain assembly. Using circular dichroism, analytical ultracentrifugation, dye-binding assays, and proximity-dependent fluorescence quenching, the authors showed that peptides with identical composition and stoichiometry could assemble into either parallel or antiparallel barrels depending solely on the register of the heptad repeat. Remarkably, “g”-register peptides assembled into parallel barrels, while “b” and “c” registers favored antiparallel arrangements.
High-resolution X-ray crystallography of the g-register assembly confirmed a parallel A3B3 αHB structure, the first such structure reported for a mixed-charge six-helix coiled-coil barrel. For the antiparallel variants, AlphaFold-Multimer models supported the experimental findings, though extensive sampling beyond default settings was necessary to produce accurate conformers. This underscores both the power and limitations of AI-based structure prediction for higher-order multimeric systems.
These findings add a new design parameter—heptad register—to the coiled-coil toolbox, complementing hydrophobic core packing and electrostatic pairing strategies. Moreover, the ability to specify helix orientation through register selection opens avenues for precision design of peptide-based nanostructures, particularly those requiring asymmetric topology, directional assembly, or channel formation.
This work not only demonstrates a rare example of conditional heterohexameric assembly but also provides an instructive model for tuning dynamic structural features in synthetic peptide systems. The implications extend beyond structural biology, offering templates for bioinspired materials, sensors, and molecular delivery systems engineered from first principles.