Oxidative Peptide Coupling
Reflecting work in the Proulx Group
In a study from Caroline Proulx's group at the North Carolina State University, published in Organic Letters, she and her colleagues address a persistent challenge in oxidative peptide coupling, maintaining side chain integrity during the formation of ketoxime linkages. Their work builds on a growing interest in using N-aryl peptides to expand the chemical space of peptide-based therapeutics.
Previous methods for oxidative couplings using N-aryl peptides performed well in aqueous buffer but encountered significant limitations when extended to more complex amino acids. Specifically, N-aryl serine, tyrosine, and tryptophan were prone to side chain loss, attributed to oxidative degradation during the reaction.
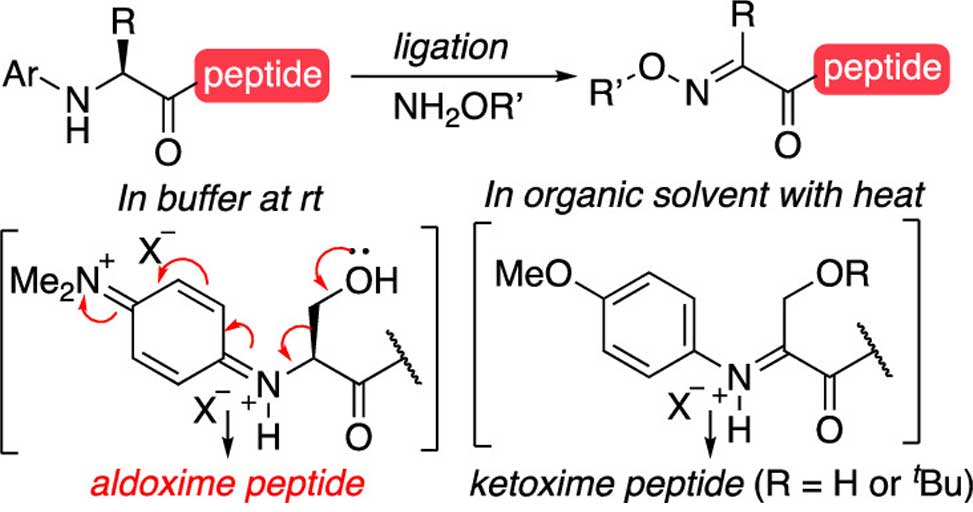
This study demonstrates that a key determinant of this reactivity is the choice of solvent. By shifting from aqueous buffer to a mixed organic solvent system, MeOH/DCE, the authors observed a pronounced shift in the oxidation pathway, favoring productive Cα-oxidation over aryl ring oxidation. This change preserved side chains that were otherwise labile under aqueous conditions. Another important factor was the electronic nature of the aryl substituent. While the highly electron-rich dimethylaminophenyl group facilitated reactivity in water, it showed reduced efficiency in organic solvents. In contrast, the less electron-rich methoxyphenyl group delivered more reliable coupling in MeOH/DCE, with excellent retention of sensitive side chains.
Group members also refined the Pd-catalyzed N-arylation of amino acid methyl esters, expanding the accessible set of N-aryl building blocks. This expanded the method’s utility across a broader range of amino acids, including Lys, Orn, Asn, Gln, Asp, Ser, Trp, Tyr, and Glu.
Together, these findings significantly expand the scope of N-aryl peptide ligations. This work broadens the synthetic toolkit for peptide and protein conjugation, offering a practical and versatile approach for building structurally diverse peptide scaffolds.
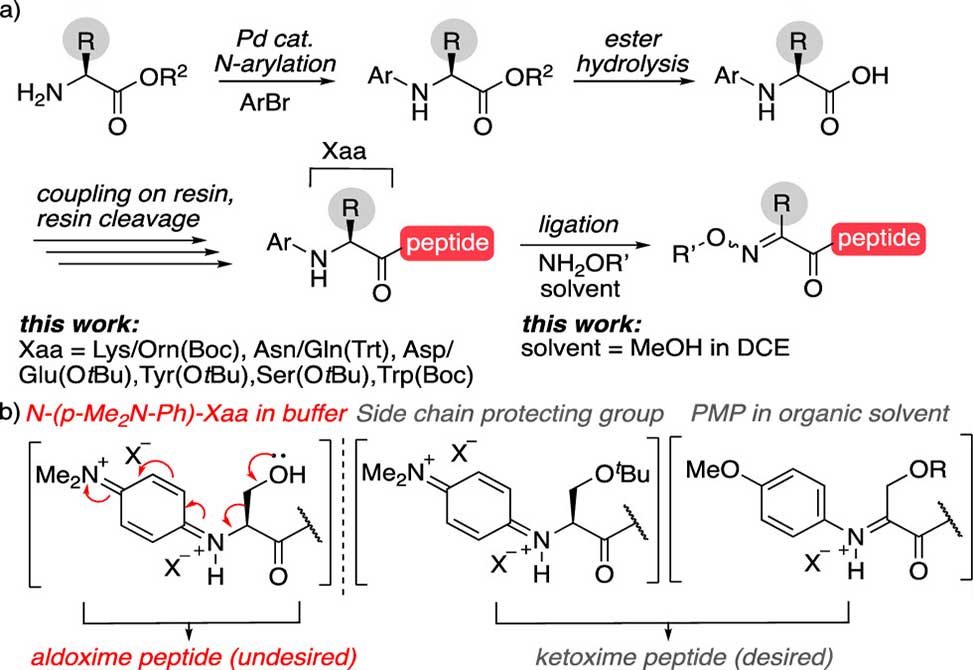
Figure 1. a| N-aryl peptide synthesis and reactivity. b| Loss of the side chain, left, can be prevented by tuning the substrate structure and reaction conditions, right. Ser is shown as a representative example.

