Mitochondrial Click
Reflecting work in the Wennemers Group
Cell-penetrating peptides, CPPs, can cross the plasma membrane and deliver diverse cargo, but directing them to specific organelles such as mitochondria remains difficult. The inner mitochondrial membrane is a particularly challenging barrier due to its dense protein composition and strong negative electrochemical potential. Mitochondria-targeting CPPs have been explored using alternating hydrophobic and cationic residues, yet achieving selective and efficient uptake is still limited.
In work published in ACS Chemical Biology, Adeline Schmitt, under the guidance of Helma Wennemers at ETH Zürich, investigated rigid polyproline II, PPII, helical scaffolds containing guanidinium-proline, Gup, residues patterned with hydrophobic groups to tune amphipathicity and uptake. They synthesized amphipathic nonapeptides of general sequence (XZZ)3, Z = Gup, where X was valine, phenylalanine, tryptophan, or cyclohexylalanine, Cha. Circular dichroism confirmed stable PPII helices with aligned hydrophobic and cationic edges. Cellular uptake in MCF-7 cells revealed that only the Cha-containing peptide achieved mitochondrial localization, while others remained cytosolic or nucleolar, whereas Arg-substituted controls lacking helicity failed to reach mitochondria, underscoring the importance of both secondary structure and hydrophobicity.
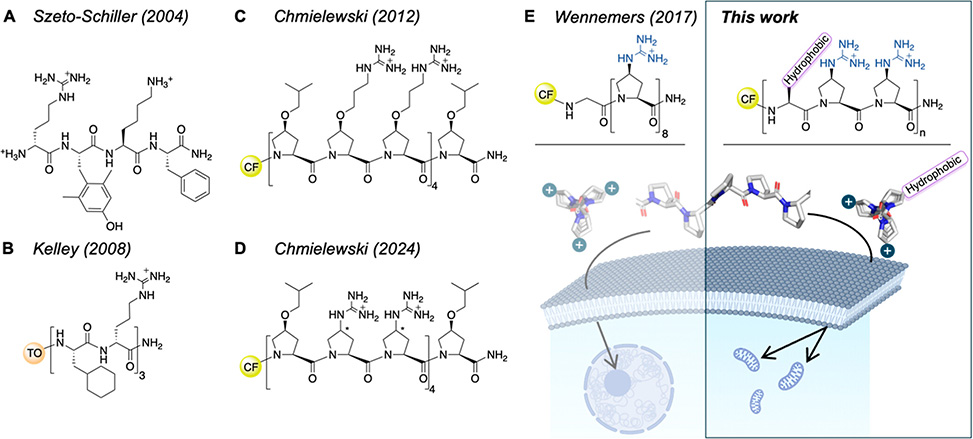
Examples of amphipathic and cationic CPPs. A| Early example of mitochondria targeting with a synthetic tetrapeptide. B| Cha- and dArg-containing mitochondria targeting hexapeptide. C| PPII-helical amphipathic 13-mer for mitochondria targeting. D| PPII-helical Gup-containing amphipathic CPP does not target mitochondria. E| Left: PPII-helical cationic Gup-based oligoproline, Z8, targeting the nucleoli. Right: Amphipathic Gup-based peptides for targeting mitochondria. Center: Structure of a PPII helical oligoproline, view along the axis and side view. CF = 5(6)-carboxyfluorescein; TO = thiazole orange; Gup = (4S)-guanidinium-proline.
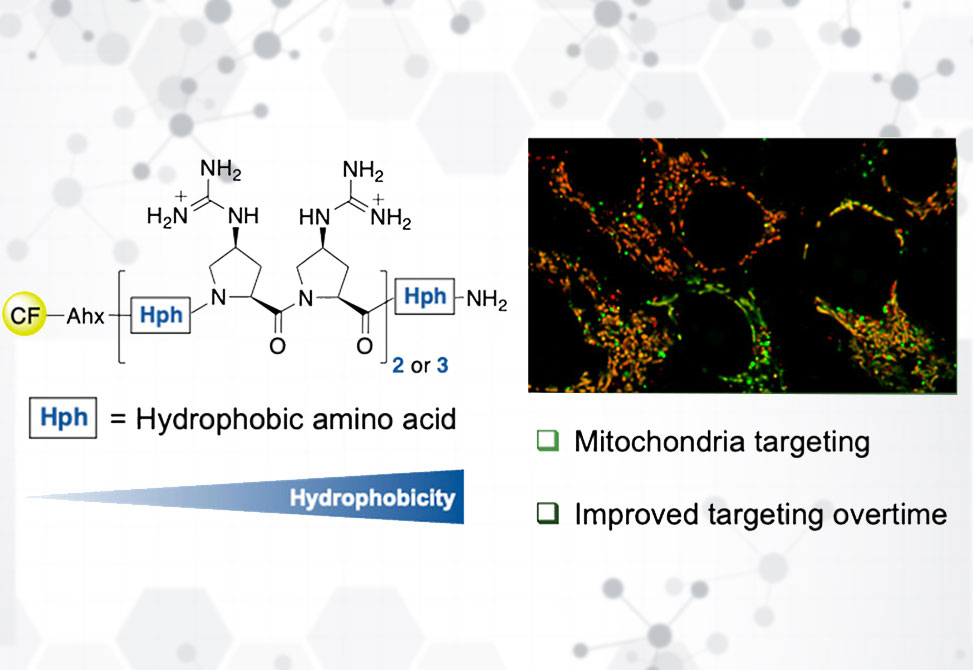
Hydrophobicity tuning further refined targeting. Adding a Cha residue at the C-terminus significantly increased uptake and mitochondrial colocalization, even in shorter 7-mer analogs. Introducing (4S)-cyclohexyl-proline, ChPro, directly into the backbone produced even more hydrophobic peptides. These variants showed higher uptake and faster membrane association, with some transitioning into mitochondria after 24 h; however, excessive hydrophobicity led to endosomal trapping, strong membrane binding, and cytotoxicity at micromolar levels.
Flow cytometry quantified these effects, showing up to 10-fold improvements in uptake upon Cha or ChPro substitution, while confocal microscopy clarified that high uptake often reflected membrane accumulation rather than true mitochondrial entry. Time-course studies revealed that several ChPro-containing peptides gradually redistributed from membranes and endosomes into mitochondria over 24 h, with stable intracellular persistence confirmed by lysate stability assays.
This work defines design rules for mitochondria-targeting CPPs: a rigid PPII backbone with guanidinium groups aligned on two edges and hydrophobic residues on the third edge enables selective uptake. Subtle increases in hydrophobicity—particularly at the C-terminus—enhance mitochondrial localization, though excessive hydrophobicity promotes off-target accumulation and toxicity. Importantly, intracellular redistribution over time proves critical, suggesting that both sequence design and temporal dynamics determine organelle selectivity.
Publication Information
Author Information

Adeline Schmitt, left, recently celebrated the successful defense of her Ph.D. in the Wennemers group at ETH Zurich. Guided by her advisor, Prof. Helma Wennemers, right, Adeline focused on the design of amphipathic proline-rich cell-penetrating peptides that selectively target mitochondria. This innovative work, published in ACS Chemical Biology and highlighted here, established a new class of peptide-based tools for probing and manipulating mitochondrial function.
Adeline’s academic path reflects a strong international and interdisciplinary trajectory. She earned her Master’s degree in Complex Systems Chemistry at the University of Strasbourg, gaining early experience in peptide science and chemical biology through projects in the laboratories of Prof. Helma Wennemers, Prof. Bradley Pentelute, and Prof. Francesco Stellacci. She then conducted her Master’s thesis under Prof. Peter Faller before returning to ETH Zurich for her doctoral studies.
In addition to her scientific contributions, Adeline has been an active advocate for women in science at ETH Zurich, helping to foster a more inclusive and supportive research community. Her achievements highlight not only the impact of her research on the field of chemical biology but also her commitment to shaping the future of the scientific community itself.

