Gelatinase Targeting
Reflecting work in the Das Group
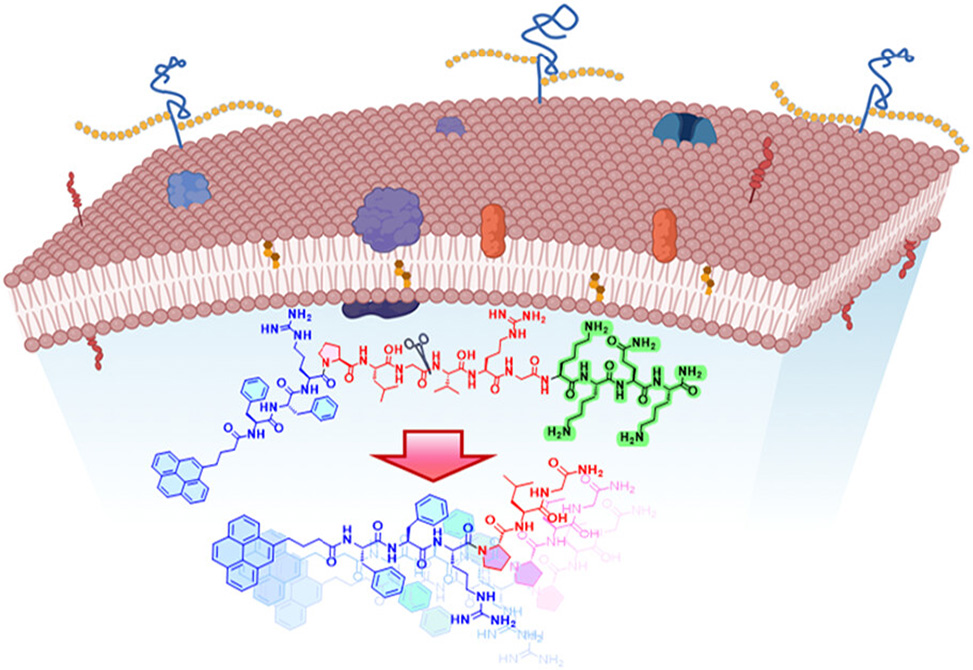
Antibiotic resistance in Staphylococcus aureus thrives on biofilm fortifications and membrane resilience, which blunt broad-spectrum drugs and damage commensal flora. A modular short peptide, Py-FGGK, answers that challenge by combining three purposeful elements into a single precision construct: a pyrene-tagged fibril-forming core, Py-FFR, a gelatinase-cleavable linker, PLGVRG, and a heparan-sulfate–binding motif, KKQK.
Published in JACS, researchers from the Indian Institute of Science Education and Research, IISER, in Kolkata, the Indian Institute of Technology, Jodhpur, and the S. N. Bose National Centre for Basic Science, Kolkata, aim to localize at infected tissue via heparan-sulfate interactions, trigger enzymatic release at the bacterial surface, and convert that release into a lethal fibril assault on methicillin-resistant S. aureus, MRSA.
The targeting step is driven by KKQK, which binds heparan sulfate on infected tissue. Isothermal calorimetry with heparin sulfate, a validated proxy, reports a formation constant of approximately 3.08 × 104 M−1, providing a quantitative anchor for site localization. In serum and buffer, Py-FGGK remains colloidally stable and shows a human-serum half-life near 4.5 h, supporting in vivo use.
Activation is delegated to gelatinase, abundant in MRSA contexts. Cleavage at the Gly-Val junction releases Py-FG, which shifts from micelles to cross-β fibrils. Pyrene serves double duty as a fluorescent reporter and a stacking promoter: excimer growth tracks aggregate formation, while microscopy confirms the morphological transition to surface-bound fibrils. Molecular simulations and spectroscopy converge on the same picture—head-on stacking by the bis-phenylalanine core, insertion at the lipid interface, and membrane thinning consistent with toroidal deformation.
The antibacterial outcome is striking. Py-FGGK achieves an MIC of 1 µM against MRSA and a 10 µM MBC, with resistance pressure limited to a two-fold MIC increase over twenty passages, contrasted with a sixteen-fold rise for ciprofloxacin under the same regimen. Membrane depolarization surpasses a gramicidin control, nucleic-acid leakage increases sharply, and propidium-iodide uptake confirms dose-dependent loss of integrity. Reactive-oxygen signals rise to levels consistent with secondary oxidative stress, reinforcing the primary membrane mechanism.
Biofilm defenses fare no better. Py-FGGK reduces surface hydrophobicity, suppresses staphyloxanthin that stiffens membranes and quenches ROS, curtails sliding motility, diminishes auto-aggregation, and cuts extracellular-polymer content in a clear dose response. The presence of the arginine moiety in Py-FGGK aids biofilm dispersion by enabling in situ nitric oxide, NO, production via ROS-mediated oxidation of arginine to citrulline within cells.In preformed biofilms, eradication approaches four-fifths of biomass at 2 × MIC; live–dead imaging shows deep penetration with extensive mortality among matrix-embedded cells.
Selectivity and safety benchmarks support translational relevance. Hemolysis remains negligible at therapeutic exposures and cytocompatibility exceeds the 70% viability bar in HEK293 and WI38 cells well above the antibacterial MIC. In fibroblast scratch assays, Py-FGGK accelerates migration, and in infected rat wounds it advances closure to roughly 90% by day 10, with histology showing re-epithelialization, vascularization, and collagen deposition that outpace controls.
The concept is elegant in its division of labor. KKQK brings the construct to the right surface, gelatinase trims the pro-peptide to an active Py-FG unit at the right moment, and the fibril-forming core executes targeted damage while sparing bystanders. The result is an enzyme-triggered, site-selective peptide therapy that couples low resistance liability with robust antibiofilm performance and tangible wound-healing benefits—an instructive blueprint for precision antimicrobials built from short, modular peptides.