Photoclick Bicycles
Reflecting work in the Lei Lab
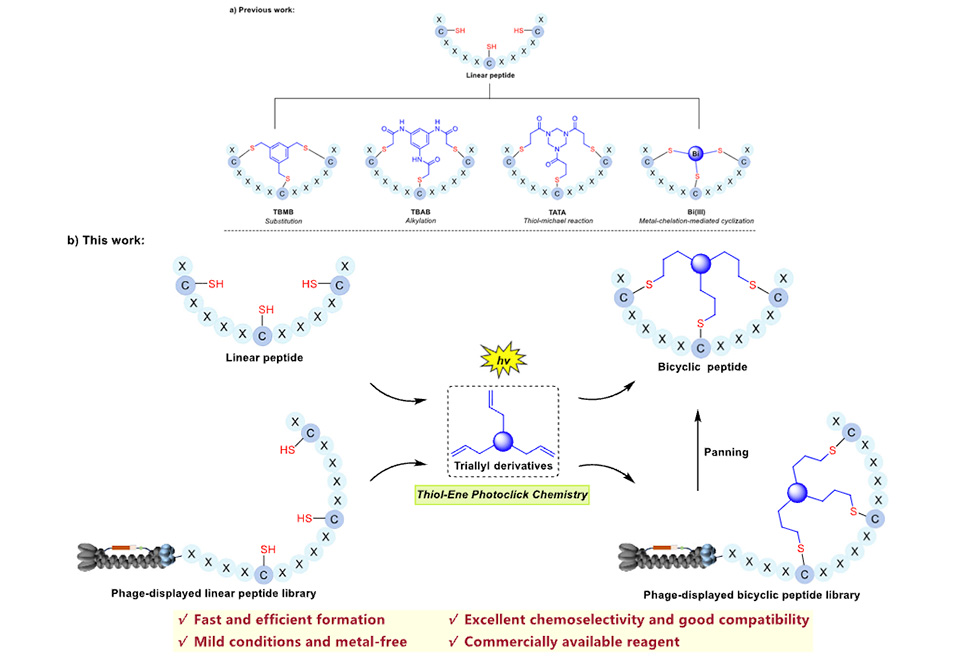
Bicyclic peptides offer exceptional promise for targeting protein-protein interactions, combining the conformational rigidity needed for high-affinity binding with improved metabolic stability. Yet their construction remains problematic. Conventional crosslinking strategies using reagents like TBMB or TBAB often trigger unwanted side reactions with nucleophilic residues, yielding complex product mixtures and limiting throughput. More recently, bismuth-based coordination methods have shown utility in phage display, but their kinetically labile metal-peptide bonds raise stability concerns in biological environments. A truly robust bicyclization platform requires covalent bond formation, broad amino acid tolerance, and compatibility with genetically encoded library technologies.
To address these challenges, a research team led by Professor Xinxiang Lei at the College of Chemistry and Chemical Engineering, Lanzhou University, China, published in Angewandte Chemie International Edition, developed the first photochemical bicyclization strategy using thiol-ene click chemistry. The approach employs TAIC, a water-soluble trifunctional crosslinker bearing three allyl groups, which reacts selectively with cysteine thiols under mild UV irradiation. Using lithium phenyl-2,4,6-trimethylbenzoylphosphinate as photoinitiator, the reaction reaches completion within six minutes in acetonitrile-water mixtures, forming stable thioether linkages across all three cysteines. Importantly, the method tolerates every proteinogenic amino acid and proceeds efficiently on unprotected peptides, eliminating the protection-deprotection cycles that complicate conventional synthesis.
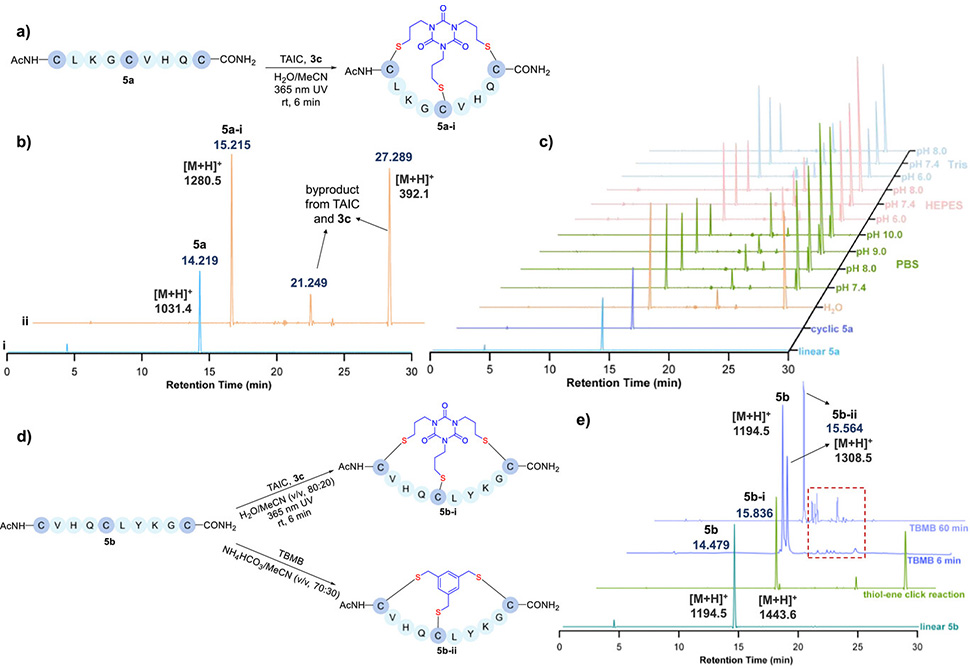
Figure 2. a| Bicyclization of linear peptide 5a via thiol–ene photoclick reaction. b| HPLC analysis, 220 nm, of peptide 5a undergoing thiol–ene photoclick reaction in H2O/MeCN, v/v, 80:20, solvent system. i| Purified starting material, linear peptide 5a. ii| Crude reaction mixture of linear peptide 5a cyclized via thiol–ene photoclick reaction. c| HPLC analysis, 220 nm, of peptide 5a undergoing thiol–ene photoclick reaction in various buffer/MeCN, v/v, 80:20, mixed solvent systems. d| Bicyclization of linear peptide 5b via thiol–ene photoclick reaction and TBMB crosslinking. e| Comparative HPLC analysis, 220 nm, of peptide 5b cyclized via thiol–ene photoclick reaction and TBMB.
Optimization studies revealed that reaction efficiency depends critically on solvent composition and pH. Acetonitrile-water systems gave superior yields compared to dimethylformamide or dimethyl sulfoxide, with the best results observed at 94 percent conversion using model substrates. When applied to full-length peptides containing strategically spaced cysteines, TAIC-mediated bicyclization proceeded cleanly under buffered conditions between pH 6.0 and 7.4, though higher pH values triggered competing side reactions between the crosslinker and photoinitiator. Direct comparisons with TBMB showed marked advantages: HPLC analysis of a peptide bearing multiple nucleophilic side chains revealed that photoclick bicyclization produced fewer byproducts and higher overall yields than alkylation-based methods. The substrate scope proved extensive, accommodating peptides with cysteine spacings ranging from one to eleven residues and yielding bicyclic products with isolated yields consistently above 60 percent.
The team then extended the strategy to phage display, engineering a peptide library on a disulfide-free pIII coat protein. After confirming that 150 micromolar TAIC and six minutes of UV exposure preserved phage infectivity, they constructed a library with approximately one billion members, each displaying a tricysteine motif flanked by randomized sequences. Panning against cyclophilin A, a validated therapeutic target involved in immune regulation and viral replication, enriched two dominant sequences after four selection rounds. The most abundant hit, designated M1, showed fivefold improved binding upon bicyclization, with the cyclic form CM1 achieving a dissociation constant of 364 nanomolar, compared to 5.09 micromolar for its linear precursor. The second hit, M2, exhibited no detectable affinity in linear form but gained submicromolar binding, 911 nanomolar, after cyclization. Plasma stability assays underscored the protective effect of bicyclization: linear M1 degraded within 30 minutes, while CM1 persisted with a half-life exceeding seven hours. NMR titration experiments using nitrogen-15 labeled cyclophilin A confirmed that CM1 binds within the catalytic pocket, inducing chemical shift perturbations in residues known to mediate substrate recognition.
These findings establish thiol-ene photoclick chemistry as a practical and scalable platform for bicyclic peptide discovery. By integrating rapid photochemical crosslinking with phage display, the method enables high-throughput screening of conformationally constrained libraries while maintaining the chemoselectivity and mild conditions required for biological compatibility. The submicromolar ligands identified against cyclophilin A demonstrate that photoclick-derived bicycles can achieve both high affinity and enhanced stability, addressing two persistent challenges in peptide therapeutics. Beyond cyclophilin A, this approach offers a generalizable route to targeting protein-protein interactions that resist small-molecule modulation, expanding the functional scope of genetically encoded peptide libraries and accelerating the translation of bicyclic scaffolds into drug discovery pipelines.