Allosteric Turns
Reflecting work in the Sampaio-Dias Lab
Disorders of the central nervous system linked to dysregulation of dopamine D2 receptors — including depression, drug addiction, Parkinson’s disease, and movement disorders — remain challenging to treat with selectivity. While most known allosteric modulators of G protein–coupled receptors are synthetic, the tripeptide melanostatin, MIF-1; Pro–Leu–Gly-NH2, stands out as the only endogenous positive allosteric modulator, PAM, with specificity for the D2 receptor. First isolated from hypothalamic extracts in 1971, MIF-1 potentiates dopamine agonist binding in an inverted-U concentration–response profile, yet its biosynthesis, bioactive conformation, and structural pharmacophore remain incompletely defined. Importantly, the l-proline residue in MIF-1 has long been suspected to play a critical role in adopting a type II β-turn conformation thought to underlie its activity.
To probe these structural determinants, researchers from the Sampaio-Dias Lab at the University of Porto, LAQV-REQUIMTE, in collaboration with colleagues from the University of Santiago de Compostela, published in ACS Medicinal Chemistry Letters, synthesized and evaluated a series of l-pipecolyl-based MIF-1 analogues, substituting proline with the six-membered pipecolic acid scaffold and introducing variations at the C-terminal functional group. The synthetic route employed standard peptide coupling, Boc deprotection, and amidation steps to yield a small focused library including compound 1, ipecolyl–Leu–Gly-NH2, and compound 9, pipecolyl–Leu–Gly-OMe, with full characterization by NMR and high-resolution mass spectrometry.
When tested in functional assays at human D2 receptors expressed in CHO cells, the analogues showed divergent effects on dopamine potency. At subnanomolar concentrations, 0.01 nM, compound 1 modestly increased dopamine potency, 1.8-fold, while compound 9 matched or exceeded the activity of MIF-1 itself, 4.1-fold. At 1 nM, MIF-1 activity declined and compound 1 lost activity altogether, but compound 9 retained robust PAM activity, shifting the dopamine EC50 more than four-fold without altering maximal efficacy. Control compounds containing carbamate substitutions were inactive, highlighting the importance of the amide-to-ester isosteric replacement in conferring activity.
Mechanistic studies confirmed that neither 1 nor 9 acted as intrinsic agonists, supporting a bona fide PAM mechanism. In silico calculations suggested that 1 preferentially adopts β-turn conformations, while 9 favors γ-turns due to the absence of a C-terminal carboxamide hydrogen bond donor. Cytotoxicity assays in differentiated SH-SY5Y neuroblastoma cells showed that both compounds were non-toxic at concentrations up to 100 μM, comparable to the parent peptide.
Taken together, this work demonstrates that homologation of proline with pipecolic acid is compatible with D2 receptor PAM activity, and that an amide-to-ester substitution can not only be tolerated but also enhance potency. The findings challenge prior assumptions that β-turn conformations are strictly required for activity and instead point to alternative structural solutions, including γ-turn motifs. By revealing flexible pharmacophore requirements for MIF-1 derivatives, this study provides valuable structure–activity relationship insights and identifies compound 9 as a promising lead for the rational design of novel anti-Parkinson’s drug candidates.
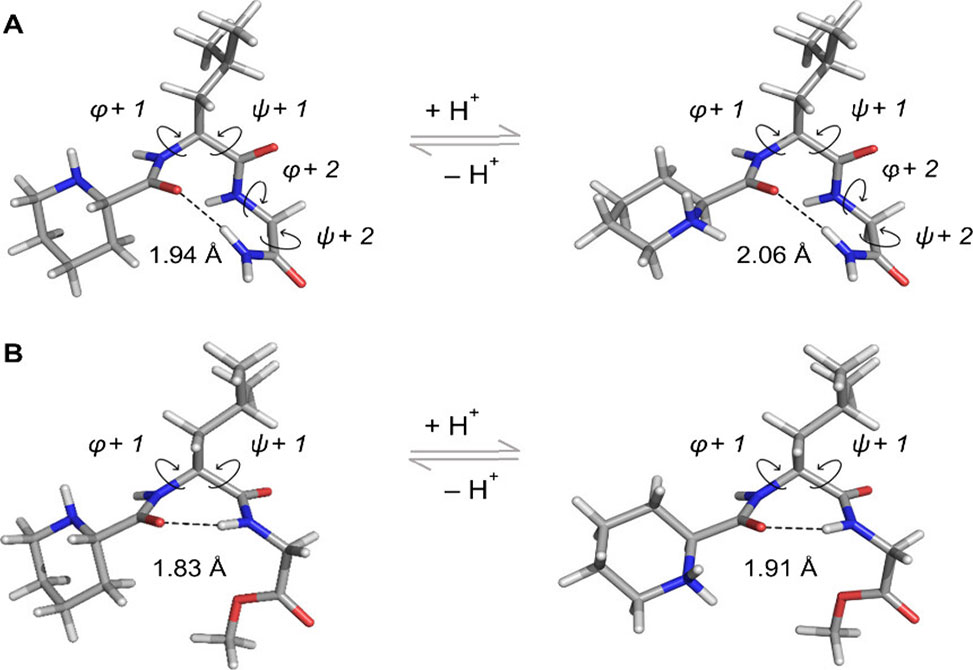
Optimized geometries of 1|A and 9|B at the PBE0/6–31G(d,p) level of theory in implicit water using the IEFPCM solvation model, exhibiting a type I′ β-turn and a classical γ-turn, respectively, assisted by intramolecular hydrogen bonds, dashed black lines.

