Cyclodepsipeptides
Reflecting recent work in the Del Valle and Ebright Groups
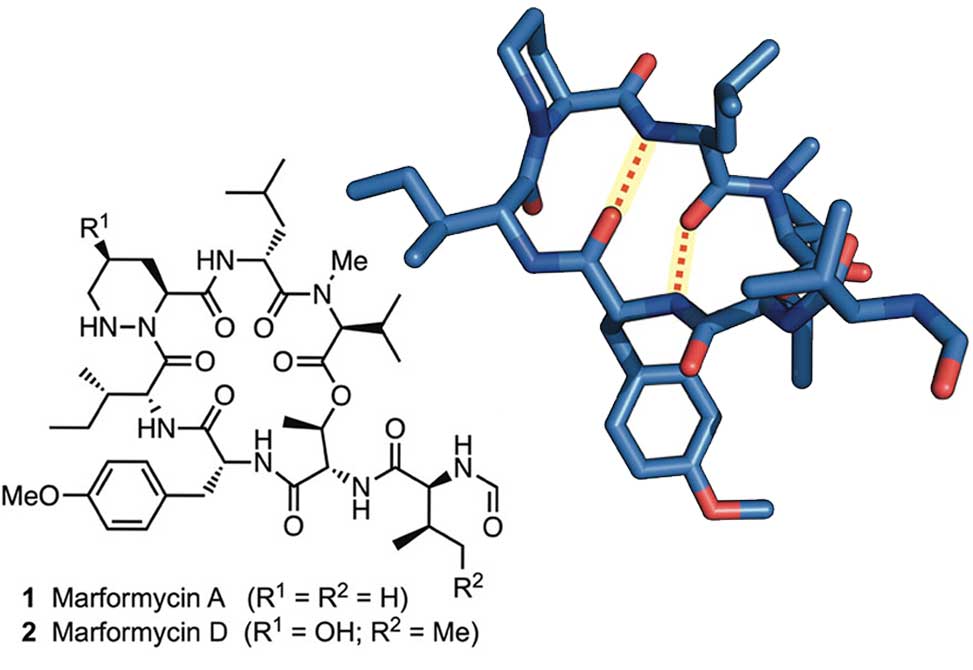
p>Members of the Del Valle Group at the University of Notre Dame, in collaboration with the Ebright Group at Rutgers, report in Organic letters, the synthesis of the antimicrobial cyclodepsipeptides marformycin A, 1 and marformycin D 2 using a solid-phase approach.

A scalable solution-phase synthesis of the γ-hydroxypiperazic acid subunit in 2, starting from cis-hydroxyproline, is also described in this work.
Structural analysis of 1 and its Leu-epi congener demonstrates conformational differences that may underlie their divergent antimicrobial activities. With an efficient synthetic route in hand, elucidation of conformation–activity relationships within this family of depsipeptides is currently underway in the Del Valle laboratory.