Antimicrobial Lipopeptides
Reflecting recent work in the Hamley Group
Chirality plays a crucial role in the self-assembly of biomolecules in nature. Peptides show chirality-dependent conformation and self-assembly. Lipidation of peptides occurs in vivo and has recently been exploited in designed conjugates to drive self-assembly and enhance bioactivity.
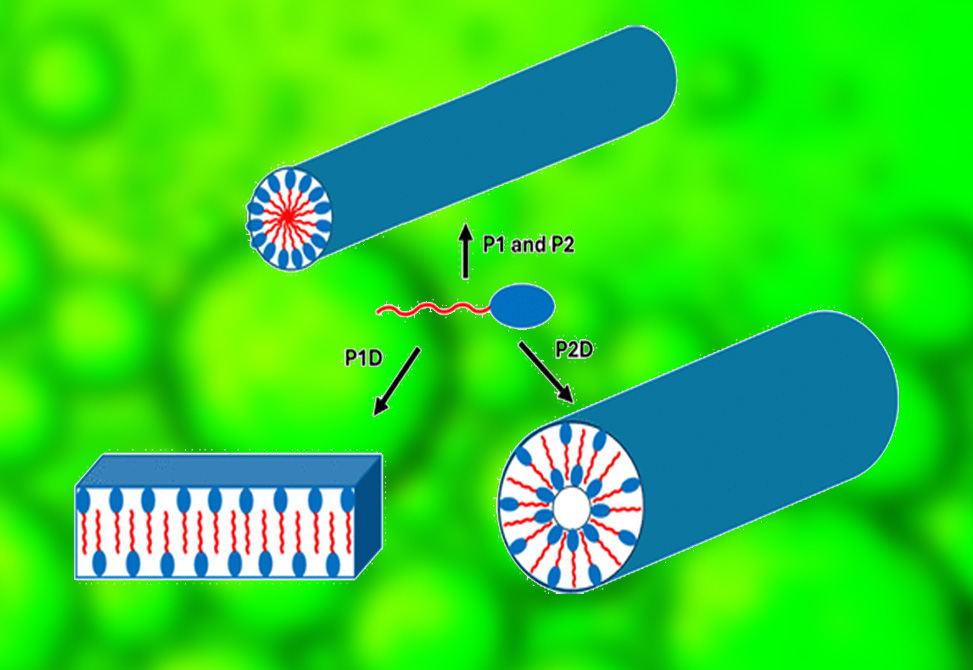
Published in ACS Applied Bio Materials, members in the Hamley Group at the University of Reading, present how a library of pH-responsive homochiral and heterochiral lipidated tripeptides has been designed. The designed lipopeptides comprise homochiral C16–YKK or C16–WKK, where all the amino acids are L-isomers, and two heterochiral conjugates C16–Ykk and C16–Wkk, where the two lysines are D-isomers.
The self-assembly of all the synthesized lipopeptides in aqueous solution was examined using a combination of spectroscopic methods along with cryogenic-transmission electron microscopy, cryo-TEM, and small-angle X-ray scattering, SAXS. Interestingly, it was observed that at acidic pH all the lipopeptides self-assemble into micelles, whereas at basic pH the homochiral lipopeptides self-assemble into nanofibers, whereas the heterochiral lipopeptides self-assemble into nanotapes and nanotubes.
A pH switch was demonstrated using a thioflavin T fluorescence probe of β-sheet structure present in the extended structures at pH 8. The group members demonstrate that both chirality and pH in lipopeptides influence the self-assembly behavior of the model tripeptides, which also show promising bioactivity. Good cytocompatibility is observed in hemolytic assays and antimicrobial activity against both Gram-negative and Gram-positive bacteria is shown through the determination of minimum inhibition concentration, MIC, and minimum bactericidal concentration, MBC, values and live/dead bacteria staining assay.