Azapeptoid Collagen
Reflecting work in the Del Valle Group
Collagen’s mechanical grace comes from a deceptively simple code: repeating Gly-Xaa-Yaa triplets that assemble into three left-handed PPII chains winding into a right-handed triple helix. Nature’s favorites at Xaa and Yaa—proline, Pro, and 4-hydroxyproline, Hyp, earn their place by pre-organizing ϕ/ψ around PPII yet pay a price: tertiary amides with a nontrivial cis population that the collagen fold must continually fight back to trans. That tension has inspired a generation of chemists to probe how far the code can be bent—swapping in peptoids, N-alkylglycines, fluorinated prolines, azaprolines—without unthreading the helix.
In a study published in Peptide Science, Wright, Rathman, and Del Valle from the University of Notre Dame, push that exploration into azapeptoids, replacing the peptoid backbone methylene, CH2R, with NHR to give N-aminoglycine, aGly, and its N'-alkylated congeners. Conceptually, azapeptoids are a clean thought experiment: they preserve the tertiary amide character that often correlates with collagen stability in peptoids, but they introduce a hydrazide N–H capable of hydrogen bonding and subtly different rotational energetics. The authors ask a pointed question: when such residues are placed at the central Xaa, position 11, of a standard (Gly-Pro-Hyp)7 collagen mimetic peptide, CMP, do they stabilize, destabilize, or simply get out of the way?
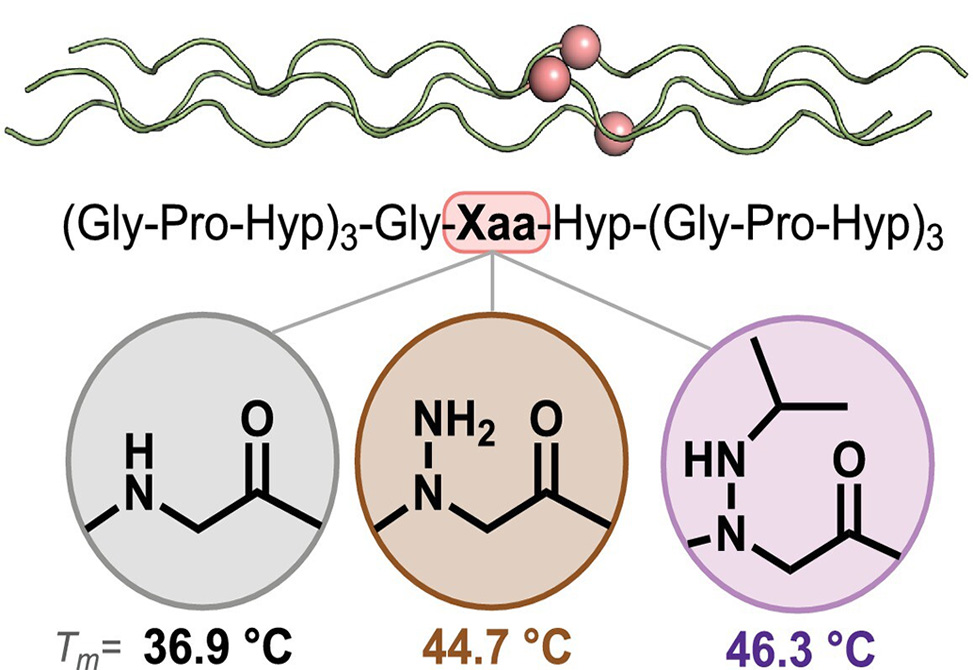
The answer is nuanced and mechanistically revealing. By circular dichroism, CD, at pH 7.4, all variants—Pro11 as the parent, aGly11, aAla11, and the canonical Gly11/Ala11 controls—display native PPII/triple-helical signatures, max ≈ 225 nm, min ≈ 204 nm. In other words, the fold tolerates the substitution. Thermodynamics is where the differences emerge. The parent Pro11-CMP melts at 53.2 °C. Swapping Pro for Gly or Ala lowers stability as expected, 36.9 °C and 46.6 °C, respectively, recapitulating the classic PPII propensities. Introducing an azapeptoid at Xaa yields split behavior: aGly11-CMP sits at 44.7 °C, a clear gain over Gly11 but still below Pro11, while aAla11-CMP drops further to 39.7 °C. That divergence echoes prior single-strand work from the same group: N-amination of Gly promotes PPII, whereas N-amination of a Cα-substituted residue tends to bias β-strand-like conformations.
To test whether hydrophobic “peptoid logic” carries over to azapeptoids, the team executes a neat late-stage N′-alkylation on aGly11-CMP, reductively appending eight different groups, isopropyl, butyl, benzyl, cyclohexyl, et cetera, directly onto the hydrazide. The CD fingerprints remain triple-helical, but the thermal map is not a simple replay of peptoid behavior. Most N'-alkylations do not raise Tm; some even depress it, for example, n-pentyl to 38.8 °C, benzyl to 40.9 °C. Two standouts, however—(iPr)aGly11-CMP and (Bu)aGly11-CMP—deliver modest, reproducible gains, ≈ +1.4–1.6 °C, to 46.3 °C and 46.1 °C, respectively, relative to aGly11. The take-home is that hydrophobic bulk alone—so successful in many peptoid CMPs—does not guarantee stabilization in the azapeptoid context; local H-bonding and subtle electronic effects of the hydrazide N–H likely reshape the balance between PPII pre-organization and unwanted cis bias.
Kinetics provide a second line of insight. The rate-determining step in collagen refolding is cis/trans isomerization of the tertiary amide. The authors previously accelerated refolding with a δ-oxaproline that lowers the isomerization barrier. By contrast, hysteresis CD here shows that neither aGly11 nor (iPr)aGly11 significantly changes the refolding rate relative to Pro11. Thus, the hydrazide subtly perturbs thermodynamics without offering a kinetic shortcut; the triple helix still pays the same isomerization toll during assembly.
Methodologically, the work is tight and modular. Standard Fmoc SPPS builds the CMPs, while submonomer tactics and on-resin activations install aGly/aAla cleanly. The N'-alkylation is executed after cleavage—an attractive late-stage handle for tuning physicochemical properties without re-synthesis. Throughout, analytical rigor, RP-HPLC, HRMS, quantitative CD melts, two-state fits, keeps the comparisons on firm ground.
What does this teach us about the collagen code? First, azapeptoids are welcome—but not neutral—guests at Xaa. The aGly backbone is thermodynamically kinder to PPII than Gly, which is encouraging for designing CMPs that need functionality at the backbone nitrogen without sacrificing the fold. Second, select N'-alkyl groups can claw back part of the stability lost relative to Pro, but the hydrazide’s H-bond donor appears to compete with the pre-organization benefits that make peptoids successful. This leads to a crisp hypothesis, and the authors’ forward look: dialkylated azapeptoids that remove the N–H should disentangle those effects—retaining favorable tertiary-amide character and hydrophobic tuning while eliminating the destabilizing H-bond.
In sum, azapeptoid residues give collagen chemists a new backbone dial: a way to functionalize the amide nitrogen, tune stability in both directions, and decouple thermodynamic and kinetic contributions to folding. The numbers matter—Pro11 53.2 °C; aGly11 44.7 °C; (iPr)aGly11 and (Bu)aGly11 ≈ 46 °C; Gly11 36.9 °C; Ala11 46.6 °C—but the concept matters more: backbone N-substitution is not merely tolerated by the triple helix; it is designable. With dialkylated variants on deck, this chemistry is poised to clarify how tertiary-amide electronics, local H-bonding, and hydrophobicity choreograph PPII pre-organization—and to expand the palette for CMP engineering in materials and medicine.