B-cell Epitope
Reflecting work in the
Therapeutic blockade of PD-1/PD-L1 signaling with monoclonal antibodies (mAbs) has shown clinical success and activity across a broad set of cancer subtypes. However, monotherapy with PD-1/PD-L1 inhibitors are only effective in a subset of patients and ongoing studies show efficacy of treatment depends on a combinatorial approach. Contrary to mAbs chimeric B-cell cancer vaccines incorporating a “promiscuous” T-cell epitope have the advantage of producing a polyclonal B-cell antibody that can potentially induce memory B- and T-cell responses, while reducing immune evasion and suppression.
Herein, researchers in the Kaumaya lab describe a novel PD-1 B-cell peptide epitope vaccine (amino acid 92–110; PD1-Vaxx) linked to a measles virus fusion peptide (MVF) amino acid 288–302 via a four amino acid residue (GPSL) emulsified in Montanide ISA 720VG that aims to induce the production of polyclonal antibodies that block PD-1 signaling and thus trigger anticancer effects similar to nivolumab.
In preclinical studies, the PD1-Vaxx outperformed the standard anti-mouse PD-1 antibody (mAb 29F.1A12) in a mouse model of human HER-2 expressing colon carcinoma. Furthermore, the combination of PD1-Vaxx with combo HER-2 peptide vaccine (B-Vaxx) showed enhanced inhibition of tumor growth in colon carcinoma BALB/c model challenged with CT26/HER-2 cells. The PD-1 or combined vaccines were safe with no evidence of toxicity or autoimmunity.
To read an update on how PD1-Vaxx is headed to Phase 1 clinical trials see this article: Br J Cancer. 2021 Mar 26.
See the following links for more information about the mechanism PD1-Vaxx and it's creator, Pravin T.P. Kaumaya
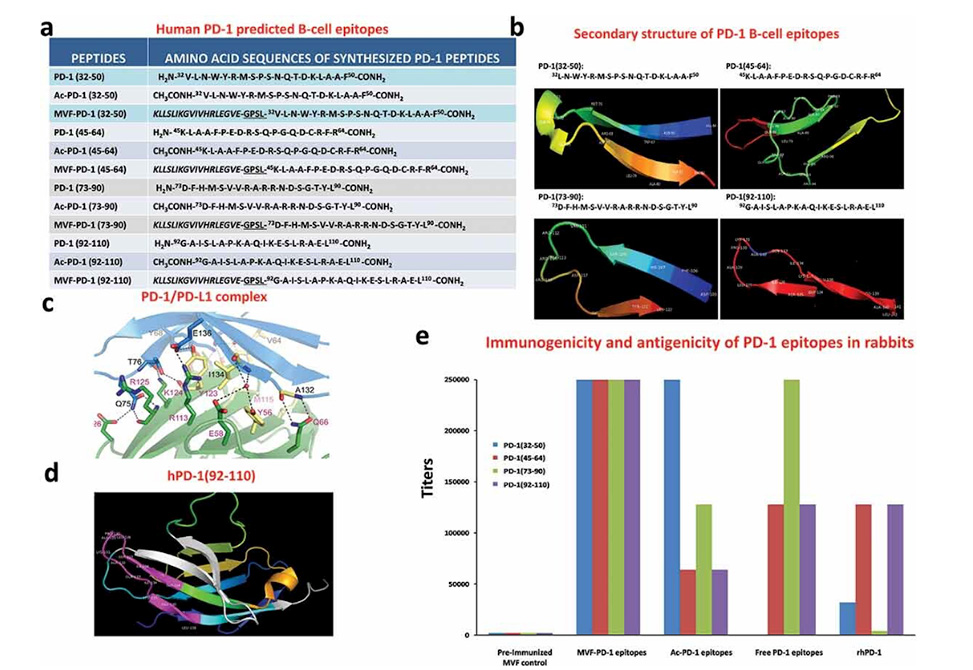
Identification of four B-cell epitope sequences of human PD-1. (a) Amino acid sequences of human PD-1, peptides 32–50, 45–64, 73–90 and 92–110 were chosen for evaluation. (b) The secondary structure of the sequences of human PD-1 epitopes as modeled by PyMOL. (c) The structure of the PD-1/PD-L1 complex as adapted by Zak et al.,Citation34 key amino acids involved in the interaction between hPD-1 (light blue ribbon model; navy blue amino acid residues) and hPD-L1 (green ribbon model; light green amino acid residues) are illustrated. Amino acids that constitute the central hydrophobic core of the hPD‐1/hPD-L1 interface are indicated in yellow. Strands on both PD-1 and PD-L1 are indicated by red letters; (d) The 3D structure of human PD-1(92–110) peptide epitope as illustrated by PyMOL. (e): Immunogenicity and antigenicity of MVF-PD-1 B-cell epitopes. New Zealand white rabbits were immunized with 1 mg of each MVF-peptide immunogens dissolved in dd H2O emulsified (1:1) in Montanide ISA 720 vehicle (Seppic) with 333 μg of N-acetylglucosamine-3yl-acetyl-l-alanyl-d-isoglutamine (nor-MDP). Rabbits were boosted with the same doses at 3-week intervals. Blood was collected via the central auricular artery in rabbits. Sera (terminal) from rabbit (3Y+3) immunized with MVF-PD-1 peptide immunogens were tested individually versus the immunogen, acetylated B-cell epitope, the free B-cell epitope and the rhPD-1 protein by ELISA. 200 ng/well peptide or 500 ng/well of rhPD-1protein were used in duplicates to coat the ELISA plates. Titers are defined as the highest dilution of sera with an absorbance value of 0.2 after subtracting the blank

