β-Hairpin Oligomers
Reflecting work in the Nowick Group
Soluble, toxic oligomers of amyloid-β, Aβ, are central to our understanding of Alzheimer’s disease, yet their molecular structures remain difficult to capture because these assemblies are heterogeneous and short-lived. Building on evidence that β-hairpins recur within Aβ oligomers, researchers in the Nowick Group at the University of California, Irvine, report in Organic & Biomolecular Chemistry a detailed structural study using macrocyclic β-hairpin peptides as controlled models of Aβ folding and assembly.
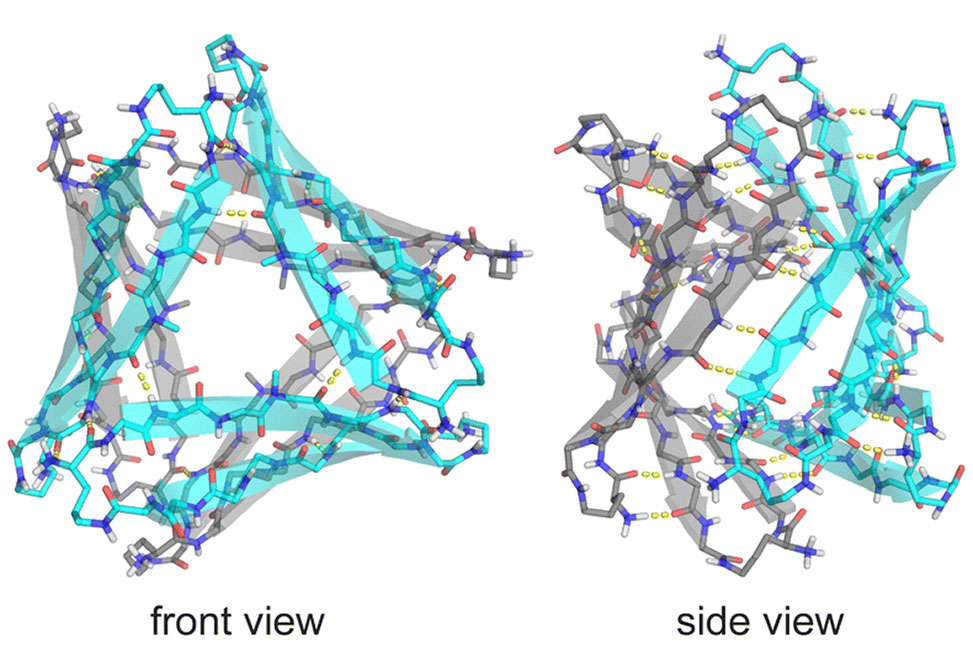
To mimic native Aβ segments, the Nowick team linked residues 16–22 and 30–36 into a β-hairpin constrained by δ-linked ornithine turns. The resulting macrocycle, peptide 1, adopted a symmetric hexamer in the crystal lattice—two triangular trimers stacked face-to-face—but in water showed no discrete state, instead forming a broad, concentration-dependent distribution of aggregates.
Replacing Phe19 with cyclohexylalanine, Cha, produced peptide 2, whose stronger hydrophobic core stabilized both crystal and solution structures. In crystals, peptide 2 reproduced the hexameric architecture of peptide 1 with a tighter, more hydrophobic interior. In solution, nuclear magnetic resonance revealed a decisive shift: two distinct oligomeric species, termed A and B, appeared in equal amounts, signaling the emergence of a well-defined assembly.
Through selective isotope labeling and multidimensional NMR, the researchers traced hydrogen-bonding and side-chain contacts that define the packing of these subunits. The data support an asymmetric hexamer built from two different trimers. Species A forms a cylindrin-like trimer with antiparallel β-strands, while species B resembles the triangular trimer seen in the crystal. The equal ratio of A and B signals, and the pattern of cross-strand nuclear Overhauser effects, confirm that the hexamer lacks symmetry and consists of intertwined A
Diffusion measurements show that two asymmetric hexamers associate into a dynamic dodecamer in solution. Molecular-dynamics simulations reinforce this model, preserving the asymmetric core while allowing flexible interfaces between hexamers. Mutational studies highlight the critical role of the Leu17–Gly33 hydrogen-bond network and precise placement of N-methyl groups in promoting order; disrupting these contacts abolishes discrete oligomer formation.
This work defines how subtle sequence edits, one hydrophobic substitution and careful backbone modification, can push an Aβ-derived β-hairpin across the threshold from amorphous aggregation to a spectroscopically resolvable, architecturally mixed oligomer. By revealing how local hydrogen bonds and hydrophobic geometry govern topology, the study provides molecular-level insight into the diversity of Aβ assemblies implicated in neurodegeneration.
Author Information

Jason Zhu was born and raised in the suburbs of Seattle where he grew up before attending Northwestern University. There, he double-majored in chemistry and chemical engineering, working in Professor John Rogers’ lab on developing wearable and implantable sensors for monitoring health. Realizing that he wanted more chemistry in his work, Jason synthesized reference compounds for the organic teaching labs and cultivated a passion for NMR spectroscopy. He enthusiastically joined the Nowick group at the University of California, Irvine, to study the supramolecular assembly of amyloidogenic and antimicrobial peptides. He hopes to utilize the skills cultivated through his Ph.D. to solve problems in the biotech industry. Outside the lab, Jason enjoys exploring new food places, playing video games, and scuba diving.

