Bicyclic Helical Peptides
Reflecting work in the Grossmann Lab
The inhibition of intracellular protein-protein interactions is challenging, in particular, when involved interfaces lack pronounced cavities. The transcriptional co-activator protein and oncogene β‑catenin is a prime example of such a challenging target. Despite extensive targeting efforts, available high-affinity binders comprise only large molecular weight Inhibitors. This hampers the further development of therapeutically useful compounds.

The Grossmann Group
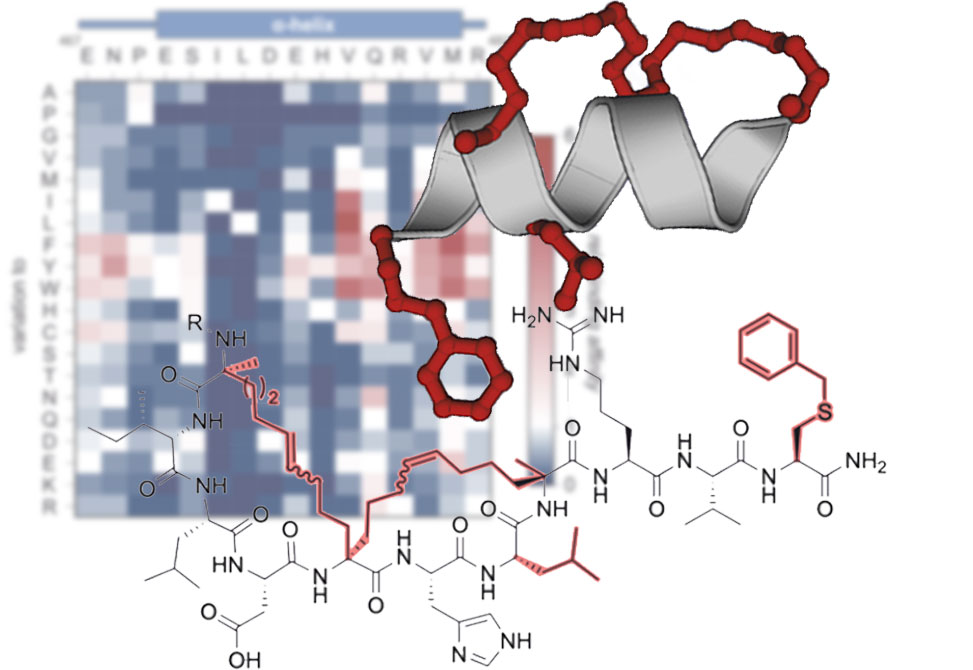
In work published in Angewandte Chemie, Intl. Ed., members from the Grossmann Lab at the Vrije Universiteit Amsterdam, report the design of a considerably smaller peptidomimetic scaffold derived from the α-helical β‑catenin-binding motif of Axin.
Sequence maturation and bicyclization provided a stitched peptide with an unprecedented crosslink architecture. The binding mode and site were confirmed by a crystal structure. Further derivatization yielded a β-catenin inhibitor with single-digit micromolar activity in a cell-based assay.
This study sheds a light on how to design helix mimetics with reduced molecular weight thereby improving their biological activity.