C–H Radiocyanation of Native Peptides
Reflecting work in the Mapp Group
In a collaborative work, published in Chemical Science, between the Mapp and Sanford groups, and researchers at the Department of Radiology, all at the University of Michigan, authors describe a net C–H radiocyanation reaction for the transformation of electron rich (hetero)aromatic substrates into 11CN-labeled products. Electrophilic C(sp2)–H iodination of the (hetero)arene with N-iodosuccinimide is followed by Cu-mediated radiocyanation with K11CN.
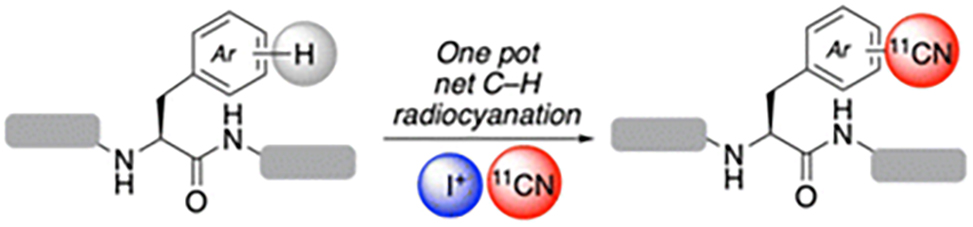
This sequence is applied to a variety of substrates, including the nucleobases uracil and cytosine, the amino acids tyrosine and tryptophan, and the peptide LYRAGWRAFS, which undergoes selective C–H radiocyanation at the tryptophan (W) residue.
This transformation is operationally simple, proceeds smoothly with peptides, and can be conducted in automated in a commercial radiosynthesis module, substrates 18-11CN and 21-11CN. It provides a straightforward approach to selectively introducing a 11CN moiety on tryptophan residues in a peptide containing multiple other aromatic amino acids without the need to synthesize dedicated labelling precursors.
Ongoing work is focused on applying this method to the 11CN-radiolabeling of various bioactive molecules of interest to the group's PET imaging program, including proteins.
The authors gratefully acknowledge The National Institutes of Health, R01EB021155, for supporting this work. Additionally they thank Dr Eric Webb and Dr Tanpreet Kaur for the preparation of K11CN solutions, Dr Allen Brooks for helpful discussions, and Dr Mónica Rivas for helping with purification of several compounds.
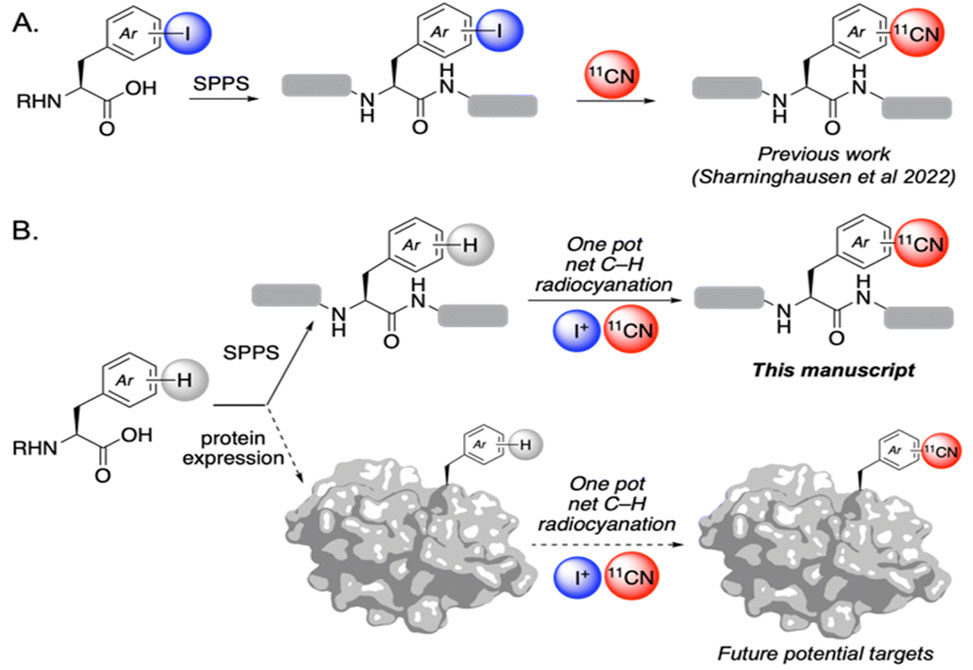
A show's the group's prior work: de novo solid phase peptide synthesis, SPPS, of iodine-containing peptides for 11CN radiolabeling.
B shows this work: net C–H radiocyanation of native peptides via electrophilic iodination/Cu-mediated radiocyanation sequence, which opens the door for ultimately labeling full proteins

