Capturing the Uncatchable
Reflecting work in the Arora Group
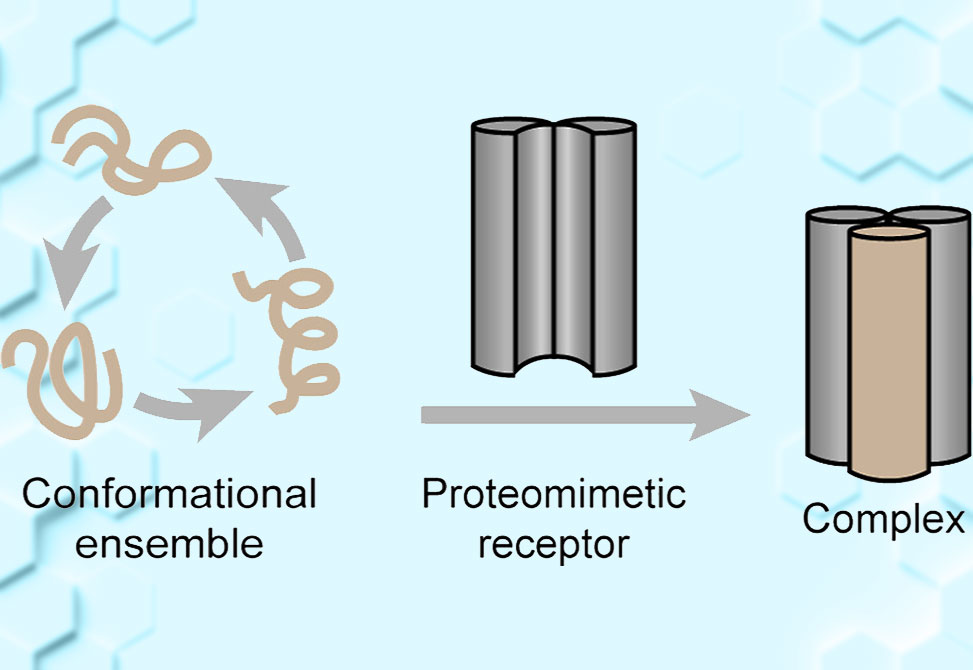
The transcription factor MYC has long stood as a prime yet elusive target in cancer biology. As an intrinsically disordered protein, IDP, MYC lacks a stable three-dimensional structure in its unbound form, making it incompatible with traditional drug discovery strategies that rely on fixed binding pockets. In a major step forward, researchers in the Arora Group at the New York University, published in JACS have now developed a rationally designed proteomimetic receptor capable of selectively trapping MYC in its biologically active conformation.
At the heart of this breakthrough is a synthetic construct called CHDMax-1, a cross-linked helix dimer designed to mimic the helical structure MYC adopts when it binds its cellular partner, MAX. Drawing inspiration from native coiled-coil interactions, the team engineered a stabilized helical interface—complete with optimized hydrophobic packing and ionic interactions—to capture MYC’s otherwise transient α-helical conformation.
In vitro assays confirmed high-affinity binding between CHDMax-1 and a MYC fragment, while in cellulo experiments demonstrated effective cellular uptake and target engagement. Confocal imaging showed nuclear localization of the fluorescein-tagged receptor in MYC-rich T24 cells. Importantly, CHDMax-1 treatment resulted in a dose-dependent increase in MYC protein levels, consistent with stabilization and protection from proteasomal degradation.
Biochemical pull-down assays using a biotin-labeled version of CHDMax-1 further confirmed selective binding to endogenous MYC. Structural modeling and cross-linking studies pinpointed the interaction to MYC’s helical binding region, reinforcing the mechanism of action proposed by the authors.
This work establishes CHDMax-1 as a proof-of-concept for a broader strategy: using cross-linked helix dimers as cell-permeable, protease-resistant scaffolds to trap IDPs that transiently adopt structured motifs. The implications extend far beyond MYC, offering a roadmap for targeting other conformationally flexible proteins implicated in disease.
Author Information

Meet Thu Nguyen, a Ph.D. candidate in Chemistry at New York University, conducting her research in the laboratory of Professor, and immediate Past President of APS, Paramjit Arora. Her work sits at the interface of chemistry and biology, with a particular focus on the design and synthesis of peptide-based molecules that modulate protein–protein interactions. By developing new strategies to interfere with these critical cellular processes, Thu’s research opens pathways toward innovative therapeutics for diseases that remain difficult to treat with traditional small molecules. Presenting this work, she earned first prize in the 2025 Dr. Elizabeth Schram Award for Young Investigator Oral Presentation at the American Peptide Society’s biennial symposium in San Diego.

