Catching Lanthanides
Reflecting work in the Zeymer Lab
Lanthanide ions offer an extraordinary toolkit for biomolecular engineering. Their long-lived luminescence enables sensitive detection, their paramagnetism provides NMR structural probes, their strong anomalous X-ray scattering aids crystallography, and their Lewis acidity and photoredox activity open catalytic possibilities. Yet natural lanthanide-binding proteins remain rare, limited mainly to alcohol dehydrogenases and the high-affinity binder lanmodulin discovered in methylotrophic bacteria. De novo design could expand this repertoire, but a fundamental challenge complicates such efforts: lanthanide ions bind promiscuously to negatively charged patches on protein surfaces, making it difficult to distinguish specific coordination from nonspecific association.
A team led by Cathleen Zeymer from the Technical University of Munich, publishing in ACS Chemical Biology, developed a screening platform that solves this discrimination problem through a dual luminescence readout. The approach exploits terbium's characteristic emission, which requires sensitization by a nearby antenna molecule that absorbs light and transfers energy to the metal. By using two antennas with different excitation wavelengths, the assay distinguishes four binding scenarios. An internal antenna, tryptophan built into the protein near the intended binding site, reports on specific coordination when excited at 280 nm. An external antenna, 2,3-dihydroxynaphthalene added to the solution, can only sensitize terbium ions accessible from the solvent and reports on surface binding when excited at 324 nm. Strong signal from both channels indicates specific binding in an accessible pocket; internal signal alone suggests a buried site; external signal alone reveals surface binding; and background from both channels means no significant coordination.
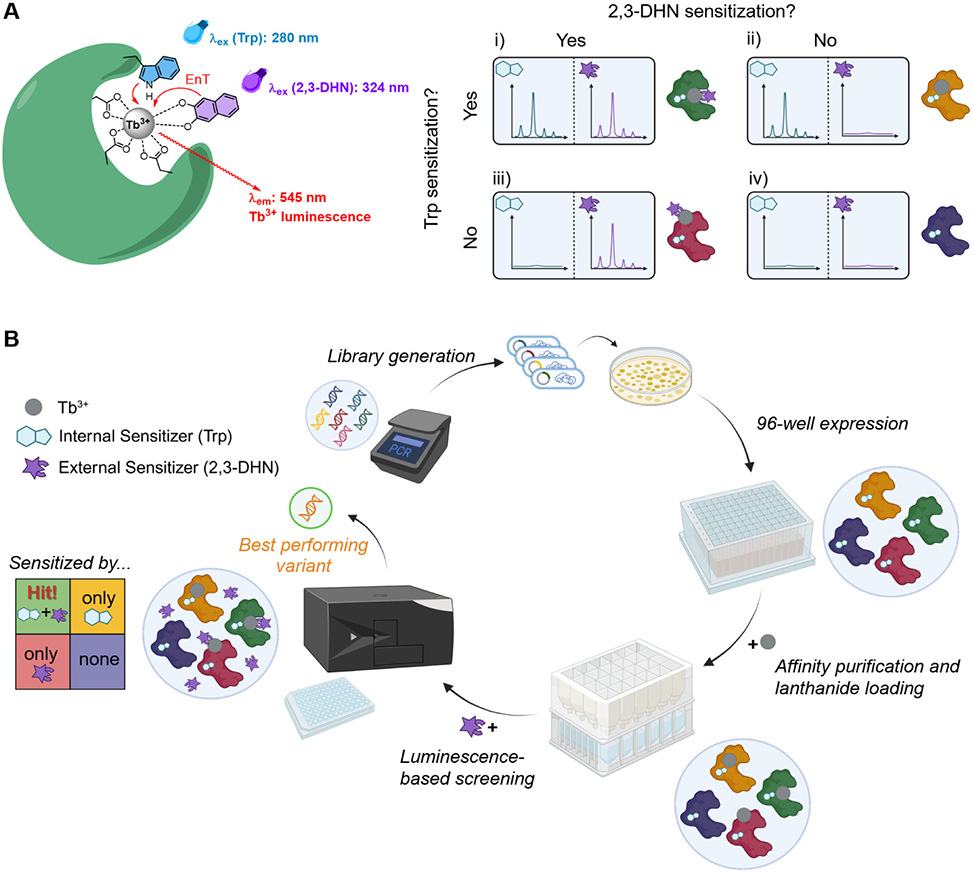
Figure 1. Design and overall workflow of the lanthanide binding assay. A| The dual luminescence readout detects the Tb3+ emission sensitized either by Trp, internal antenna, or 2,3-DHN, external antenna. B| General procedure to screen recombinantly expressed protein and peptide libraries for lanthanide binding. EnT─Energy transfer. Parts of this figure were designed using BioRender; Panel A|, Panel B|.
The workflow integrates expression, purification, and detection in 96-well format. Proteins expressed in E. coli with His-tags are captured on commercial nickel-affinity filter plates, loaded with terbium on-resin, washed to remove unbound metal, and eluted for time-resolved luminescence measurement. Validation with control proteins confirmed the platform's discriminating power. The lanthanide-dependent alcohol dehydrogenase PedH showed strong signal in both channels, while an active-site mutant lacking coordinating aspartates gave only background. Lanmodulin, which coordinatively saturates its bound lanthanide, produced internal signal but no external antenna response, exactly as predicted for an inaccessible binding site.
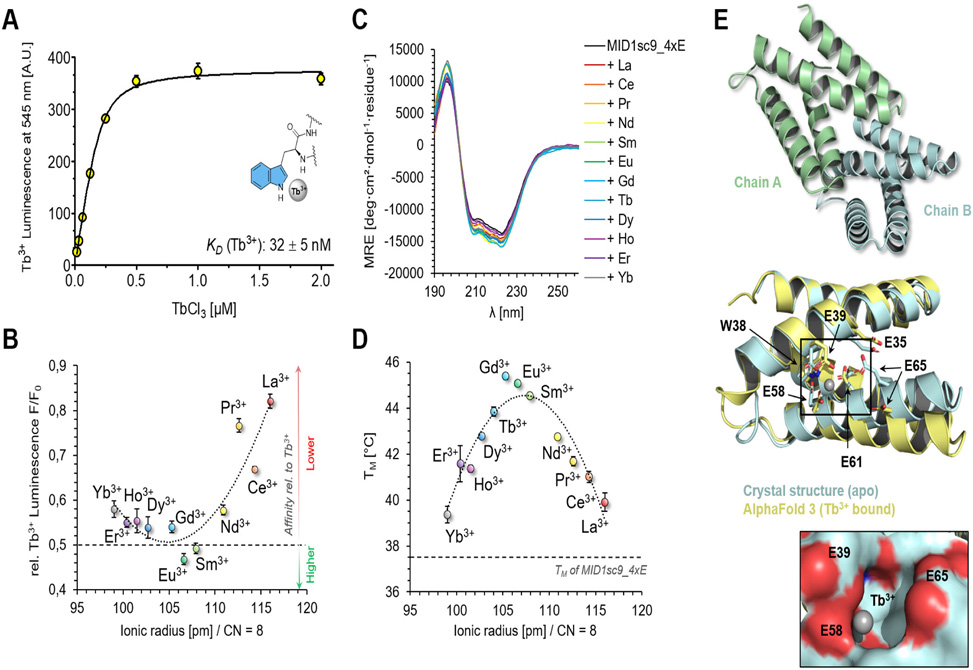
The assay proved sensitive enough to detect lanthanide binding by short peptides even when fused to the large maltose-binding protein expression tag. Trp-zipper peptides engineered with aspartate and glutamate coordination sites showed 10-fold signal above background despite only micromolar affinity. Encouraged by this sensitivity, the team constructed the MID1sc_DE library by shuffling mutations between two starting scaffolds of the helical bundle protein MID1, generating a theoretical diversity spanning from 64 to over 250,000 variants. Screening 400 representatives from this library, with four positions targeted for aspartate or glutamate replacement, identified variants with nanomolar terbium affinity. The best performer, MID1sc9_4xE, bound terbium with a dissociation constant of 32 nM and showed 100,000-fold selectivity over calcium. Intriguingly, all variants preferred medium-sized lanthanides like europium and samarium over larger or smaller members of the series. Lanthanide binding also rescued thermostability: the melting temperature of MID1sc9_4xE jumped from 37.5 °C in the apo form to 43.9 °C when terbium-bound.
Adding phosphate as a competing chelator provided tunable stringency control, enabling discrimination between nanomolar and micromolar binders within the same screen. This refinement should prove valuable for directed evolution campaigns seeking progressively tighter binding. By combining recombinant expression with a dual-channel readout that distinguishes specific from surface coordination, this platform removes a longstanding bottleneck in lanthanide-protein engineering for applications spanning MRI contrast agents, artificial metalloenzymes, and rare-earth recycling.
Publication Information
Author Information

Robert Klassen is a doctoral researcher at the Department of Protein Chemistry at the Center for Functional Protein Assemblies of the Technical University Munich, where he works under the supervision of Professor Cathleen Zeymer. He received his master's degree in biochemistry from the Martin-Luther University Halle-Wittenberg. His current research topic focuses on artificial metalloproteins, especially the identification and characterization of lanthanide-binding de novo scaffolds.

