CGRP-Responsive Receptor
Reflecting work in the Sexton and Wootten Labs
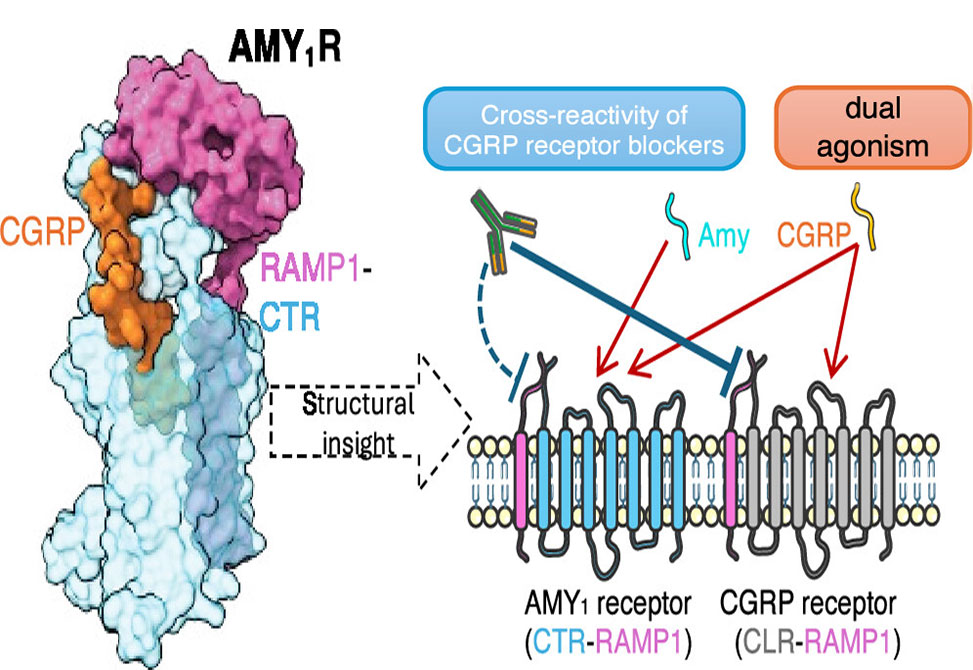
Inhibition of calcitonin gene-related peptide, CGRP, or its cognate CGRP receptor, CGRPR, has arisen as a major breakthrough in the treatment of migraine. However, a second CGRP-responsive receptor exists, the amylin, Amy, 1 receptor, AMY1R, yet its involvement in the pathology of migraine is poorly understood. AMY1R and CGRPR are heterodimers consisting of receptor activity-modifying protein 1, RAMP1, with the calcitonin receptor, CTR, and the calcitonin receptor-like receptor, CLR, respectively.
In research published in Biochemistry, members from the Sexton and Wootten Labs, present the structure of AMY1R in complex with CGRP and Gs protein and compare it with the reported structures of the AMY1R complex with rat amylin, rAmy, and the CGRPR in complex with CGRP. Despite similar protein backbones observed within the receptors and the N- and C-termini of the two peptides bound to the AMY1R complexes, they have distinct organization in the peptide midregions - the bypass motif - that is correlated with differences in the dynamics of the respective receptor extracellular domains. Moreover, divergent conformations of extracellular loop, ECL3, intracellular loop, ICL2, and ICL3 within the CTR and CLR protomers are evident when comparing the CGRP bound to the CGRPR and AMY1R, which influences the binding mode of CGRP.
These findings suggest that CGRP activation of the AMY1R occurs via a partially differentiated mechanism relative to interaction and activation of the canonical CGRPR. Nonetheless, the structural conservation of the ECD heterodimeric cleft between the two receptors and the conserved interactions of the peptide C-termini with the CGRPR and AMY1R are likely responsible for the cross-reactivity of clinical CGRPR blockers across both receptors.