Clicking Safely
Reflecting work in the Jain Group
The 1,2,3-triazole ring has become indispensable in peptidomimetic design. Its geometry and electronic properties closely mimic the trans-amide bond: similar planarity, comparable dipole moment, and preserved hydrogen-bonding capacity. Triazole-containing compounds already appear in clinical antibiotics like tazobactam and cefatrizine, and researchers continue to explore triazole-modified amino acids as building blocks for stable, bioavailable therapeutics. Yet synthesizing these hybrids presents practical hazards. Traditional routes require handling organic azides, compounds notorious for their explosive potential. Copper-catalyzed alkyne-azide cycloadditions can also suffer from Glaser homocoupling, where terminal alkynes dimerize instead of clicking with their intended partners.
A team led by Rahul Jain at the Department of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Nagar, India, published in Organic Letters, developed a two-step protocol that circumvents both problems. The sequence begins with Sonogashira coupling: a palladium-copper system joins trimethylsilylacetylene to iodophenylalanine at room temperature, installing a protected alkyne handle in 95% yield. Rather than isolating this intermediate, the team simply evaporates the solvent and advances directly to the key transformation. Under microwave irradiation, three reactions proceed in a single vessel: potassium fluoride strips the trimethylsilyl group to unmask the terminal alkyne, sodium azide and copper iodide generate an organic azide in situ from an aryl or alkyl halide, and the resulting azide immediately undergoes [3+2] cycloaddition with the alkyne. The entire multicomponent sequence completes in 25 minutes at 70 °C.
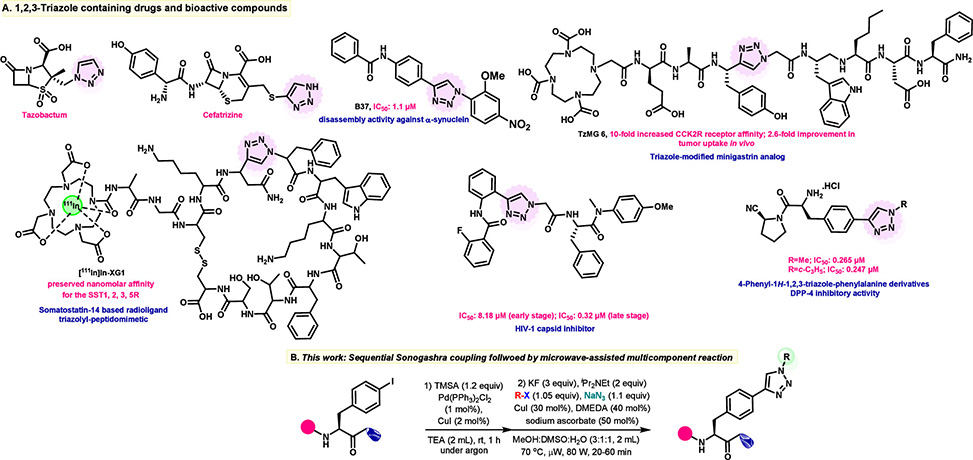
Figure 1. A| Selected examples of 1,2,3-triazole containing commercial drugs and bioactive compounds; B| Present work
Systematic optimization identified the critical variables. Copper iodide outperformed other copper salts, potassium fluoride proved essential for clean desilylation, and a solvent mixture of methanol, DMSO, and water delivered the best yields. Under these conditions, 4-tert-butyliodobenzene clicked with the phenylalanine alkyne to furnish the triazole hybrid in 81% yield. Chiral HPLC confirmed that the product retained 98.4% enantiomeric excess, demonstrating that the microwave conditions preserve stereochemical integrity. Two-dimensional NMR spectroscopy verified exclusive formation of the 1,4-disubstituted regioisomer. The substrate scope proved remarkably broad. Aryl iodides bearing electron-donating and electron-withdrawing groups reacted efficiently, as did aryl bromides, heteroaromatic halides like 3-iodoquinoline, and simple alkyl halides including methyl iodide. The protocol even tolerated the in situ generation of methyl azide, a notoriously hazardous species that chemists normally avoid handling directly.
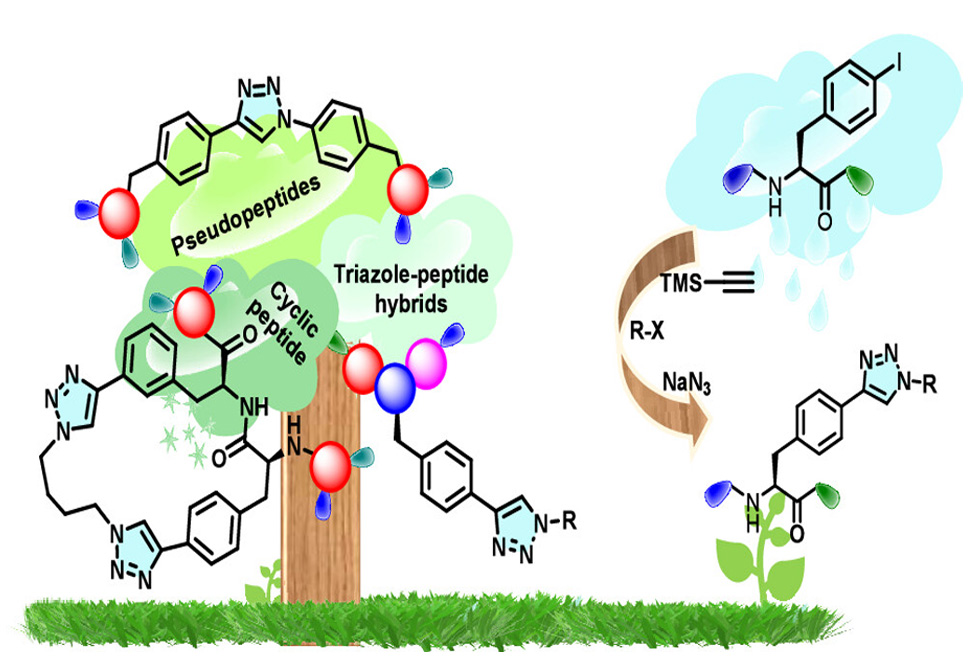
The team pushed the methodology further. Coupling iodinated amino acids produced triazole-linked pseudopeptides in 44–58% yield. Bifunctional linkers like 1,4-diiodobenzene enabled synthesis of bis-triazole derivatives bridging two phenylalanine units. Iterative growth sequences built extended ditriazole frameworks. Most impressively, the protocol modified intact peptides: di-, tri-, and tetrapeptides containing iodophenylalanine underwent clean triazole installation in 41–61% yield. When applied to a dipeptide bearing two iodophenylalanine residues, treatment with 1,4-dibromobutane produced a macrocyclic peptide featuring two triazole linkages and a butylene bridge, demonstrating the potential for constrained peptidomimetic architectures.
By generating azides only at the moment they react, this approach eliminates the storage and transfer of explosive intermediates. The microwave acceleration, single-pot execution, and broad functional group tolerance make the method practical for building triazole-modified amino acids and peptides at scale. As peptidomimetics continue gaining traction in drug discovery, synthetic routes that balance efficiency with safety will prove increasingly valuable.
Publication Information
Author Information

Karuna Thakare is a fourth-year Ph.D. candidate in the Department of Medicinal Chemistry at the National Institute of Pharmaceutical Education and Research, NIPER, S.A.S. Nagar, India, where she works under the supervision of Professor Rahul Jain. Her research focuses on innovative synthetic methodologies for the synthesis of modified amino acids and late-stage peptide modifications, with an emphasis on sustainable and efficient approaches.

