Coiled Coils
Reflecting work in the Price
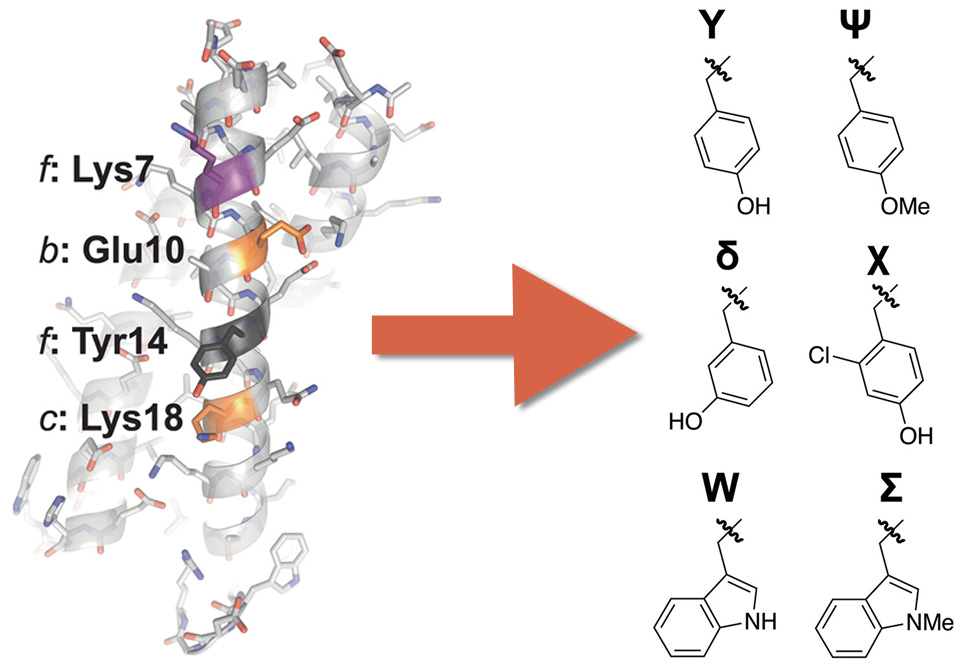
Coiled coils are one of most common protein quaternary structures and represent the best understood relationship between amino acid sequence and protein conformation. Whereas the roles of residues at the canonical heptad positions the a, d, e, and g are understood in precise detail, conventional approaches often assume that the solvent-exposed b-, c-, and f- positions can be varied broadly for application-specific purposes with minimal consequences.

The Price group
However, a growing body of evidence suggests that interactions among these b, c, and f residues can contribute substantially to coiled-coil conformational stability. In the trimeric coiled coil described in work by members of the Price Lab at Brigham Young University, published in Peptide Science, group members find that b-position Glu10 engages in a stabilizing long-range synergistic interaction with c-position Lys18, (ΔΔΔGf=−0.65±0.02kcal/mol). This favorable interaction depends strongly on the presence of two nearby f-position residues: Lys 7 and Tyr14.
Extensive mutational analysis of these residues in the presence of added salt versus denaturant suggests that this long-range synergistic interaction is primarily electrostatic in origin, but also depends on the precise location and acidity of a side-chain hydrogen-bond donor within f-position Tyr14.