Constrained Peptides
Reflecting work in the
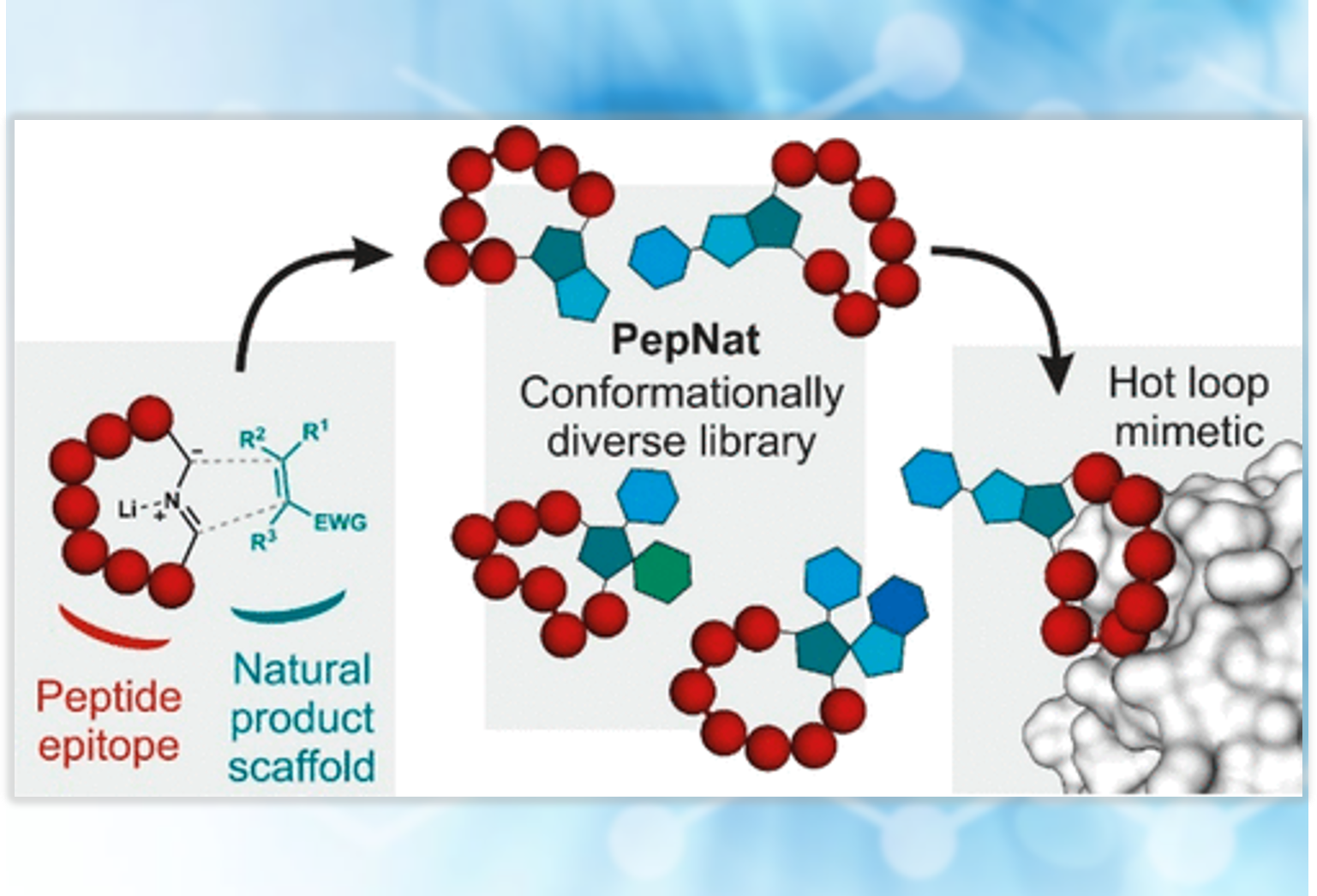
In the ongoing search to better control protein-protein interactions (PPIs), peptide sequences displaying the “omega (Ω) loop” has gained interest. An omega loop structure denotes a peptide whose C and N-terminal ends are spatially positioned near each other. However, short peptides generally lack a conformationally constrained structure.
Guéret and colleagues describe a versatile method for inserting chiral motifs between the ends of omega loops. Peptides are synthesized using traditional SPPS, followed by macrocyclization via benzaldehyde attachment and imine formation. The imine enables pyrrolidine synthesis via 1,3 dipolar cycloaddition to create four contiguous chiral centers. A variety of PepNats were synthesized that target the protein-protein interaction between inducible nitric oxide synthase (iNOS) and SPRY domain-containing SOCS box protein 2 (SPSB2). The authors found that several of the conformationally constrained peptides have a greater than 20-fold higher affinity than the unconstrained macrocyclic peptide. As a consequence, PepNats may find utility as inhibitors of protein-protein interactions.