Controlling Stereoselectivity
Reflecting work in the Wennemers Lab
Over the past two decades, proline and proline-based peptides have been established as versatile catalysts to access, via an enamine intermediate, α-functionalized carbonyl compounds. Achiral linear and symmetric β-branched aldehydes are widely used substrates and react readily with a variety of electrophiles. In contrast, β-branched aldehydes with two different substituents at Cβ have rarely been used.
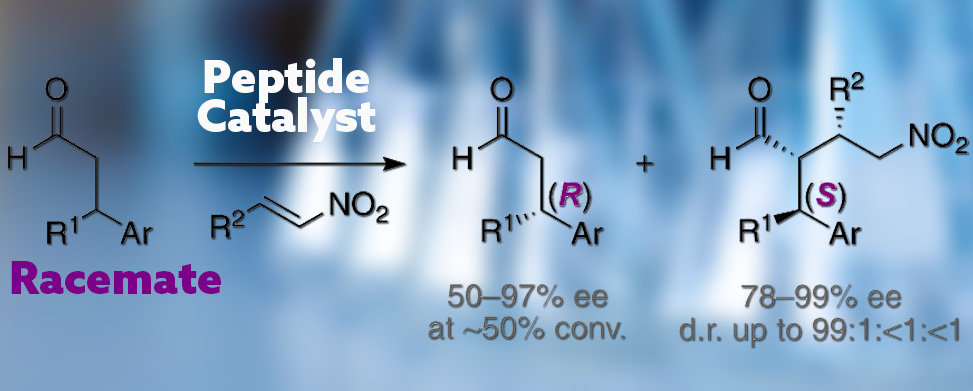
Addition reactions with control over the stereochemistry of all stereogenic centers require either the use of enantiomerically enriched starting aldehydes or the kinetic resolution of racemic aldehydes by the catalyst. The latter approach is convenient but challenging since the catalyst needs to distinguish the stereochemistry at a remote center that is connected to the enamine intermediate by a rotatable bond. In fact, previous examples of kinetic resolutions with racemic β-branched aldehydes proceeded with moderate selectivity or required a specific aldehyde with an additional stereogenic center.
In a study from the Wennemers Lab at ETH, Zūrich, published in JACS, group members present an organocatalytic kinetic resolution to yield enantiomerically enriched β-branched aldehydes and γ-nitroaldehydes with three consecutive stereogenic centers in high yields and stereoselectivities. The key to the kinetic resolution is the peptide catalyst H-DPro-αMePro-Glu-NH2. This chiral secondary amine forms diastereomeric enamines with distinctly different chiral environments, reminiscent of the chiral pockets of enzymes, that react at different rates with the electrophile.
Greta Vastakaite, Helma Wennemers, and Alena Budinská.

