Covalent Peptide Libraries
Reflecting work in the Bode Lab
Covalent inhibitors provide potent and durable target engagement, with approved drugs such as ibrutinib and osimertinib validating the approach. These compounds exploit electrophilic warheads to irreversibly or reversibly engage nucleophilic residues, most often cysteine. Despite advantages in potency and pharmacodynamics, covalent inhibitors are limited by toxicity and off-target promiscuity, creating demand for new chemistries and high-throughput methods to generate selective libraries.

The Bode Group at ETH Zürich.
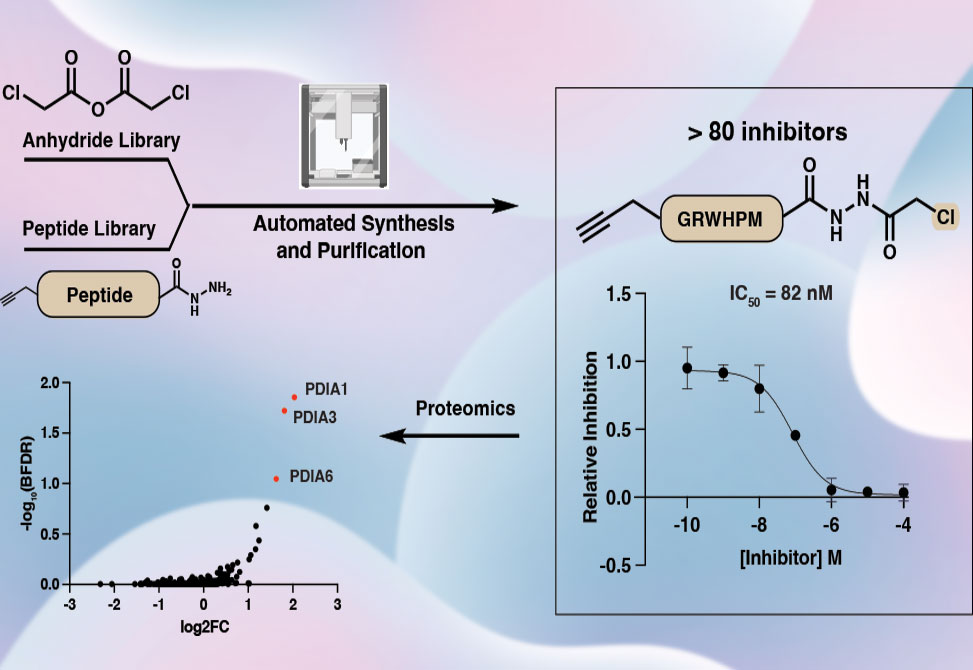
Motivated by this gap, researchers in the Bode Group at ETH Zürich, published in ACS Chemical Biology, developed a streamlined workflow for postassembly modification of peptide acyl hydrazides, enabling site-specific installation of electrophilic warheads. Using automated liquid handling and solid-phase ion exchange purification, synthetic peptides were converted directly into warhead-bearing inhibitors without laborious chromatographic purification. This platform produced 88 peptide-derived probes in 96-well format, incorporating warheads spanning chloroacetamides, Michael acceptors, alkynes, and nitriles.
As a test case, inhibitors were designed against cathepsin S, Cat S, a cysteine protease implicated in cancer and autoimmune disease. Peptides derived from selective fluorogenic substrates were synthesized and modified with electrophiles. Screening revealed that truncated peptides bearing chloroacetyl warheads displayed nanomolar inhibition of Cat S, with potency gains of up to three orders of magnitude compared to acetylated controls. IC50 determinations and kinetic analysis confirmed strong covalent engagement with catalytic cysteine residues.
Chemoproteomic profiling, however, uncovered unexpected off-target activity. Biotinylated chloroacetyl probes strongly labeled protein disulfide isomerase A1, PDIA1, and related isomerases in lysates, consistent with their high cysteine content and abundance in the endoplasmic reticulum. Control experiments confirmed that linker and biotin elements contributed significantly to this off-target effect. These findings highlight both the utility and the pitfalls of reactive electrophiles in chemoproteomic probe design.
This study demonstrates a robust, automated platform for rapid covalent inhibitor library generation. The approach accelerates discovery of selective inhibitors while also revealing liabilities, particularly unintended PDIA1 engagement. The results underscore the need for careful warhead selection and off-target characterization in covalent drug discovery.
Author Information

Meet Shaun O’Hare. Born and raised in Zürich, he completed his elementary and secondary education there before pursuing a B.Sc. in Chemistry with a minor in Biology at the University of Zürich. He went on to earn an M.Sc. in Medicinal Chemistry at the University of Copenhagen, where his master’s thesis was supported by the Novo Nordisk International Talent Grant and carried out in the Bode Group at ETH Zürich.
Following his master’s degree, Shaun returned to Zürich to begin his Ph.D. in the Bode Group, completing the research that originated in his master’s project and was ultimately published in ACS Chemical Biology. Alongside his scientific training, he also earned an MBA from the Quantic School of Business and Technology, expanding his expertise at the intersection of science and business.

