Crossing Cell Barriers
Reflecting work in the Meldal Group
The cell membrane stands as biology's most formidable gatekeeper. Large molecules, from therapeutic proteins to diagnostic agents, cannot simply walk through. This barrier has frustrated drug developers for decades, leaving countless intracellular targets, including protein-protein interactions in the cytosol, effectively undruggable. Cell-penetrating peptides, CPPs, offer one promising solution: short sequences that ferry cargo across membranes through mechanisms still being unraveled. Yet even the best CPPs often deliver cargo inefficiently, trapping it in endosomes rather than releasing it to the cytoplasm where it can act.
A team led by Nobel Laureate Morten Meldal at the University of Copenhagen, published in Angewandte Chemie, Int. Ed., reasoned that modifying the proline residue in cyclic CPPs could dramatically improve their penetration. Proline naturally induces β-turns, the structural kinks that help peptides interact with membranes. The researchers designed four novel derivatives of trans-4-hydroxy-L-proline, each bearing either an ether or secondary amine linkage to a hydrophobic group: a flat, aromatic naphth-2-ylmethylene or a flexible dodecyl chain. They incorporated these building blocks into sixteen peptide constructs, including linear sequences and three different click-cyclized scaffolds, then systematically compared their ability to enter MCF-7 breast cancer cells.
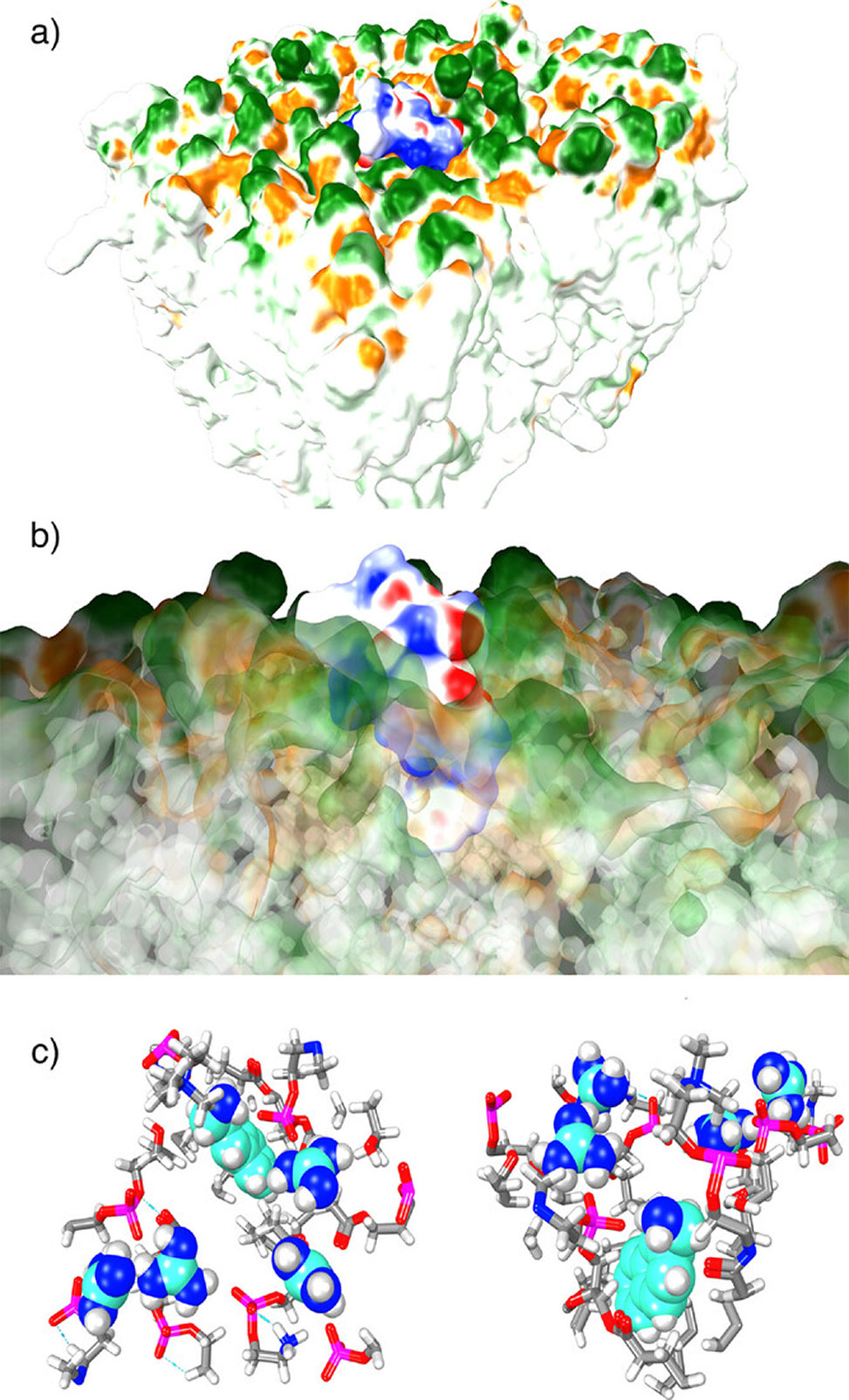
Figure 4. The interaction of cCPP 15 with the cell membrane, as assessed through molecular dynamics in water, MOE, 5 ns, between the cyclic peptide and a mammalian cell membrane. a| View from the outside of the cell of the embedded cCPP 15. b| The interaction of the naphthalene moiety with the hydrophobic membrane interior. c| Top view and side view of the interaction of the arg guanidines and the napht-2-ylmethylamine groups with the phosphate diesters of the membrane headgroups, with other parts of the molecules excluded.
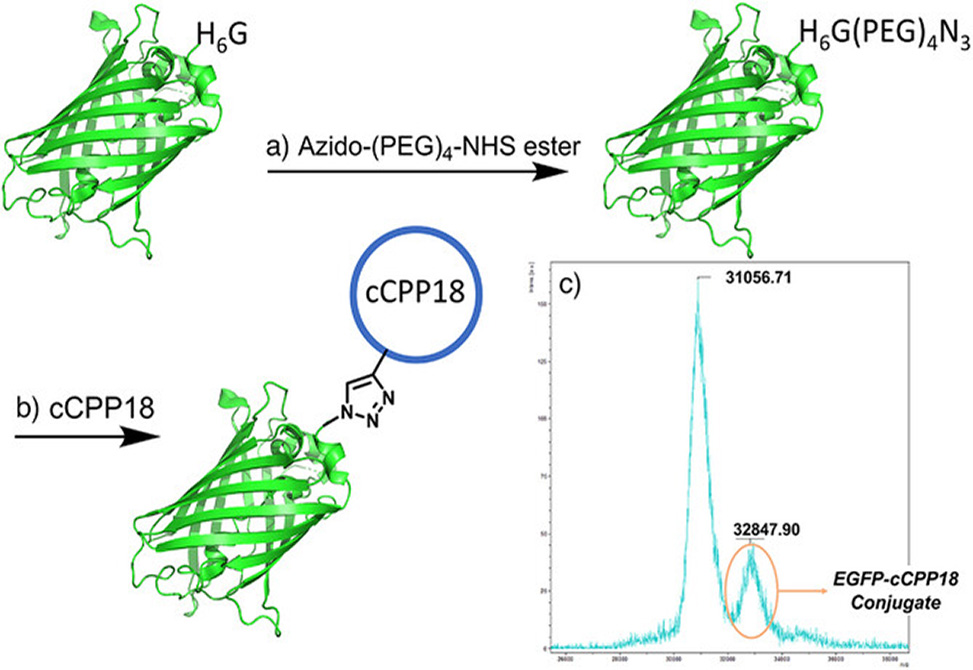
Flow cytometry and spinning disk confocal microscopy revealed stark differences among the variants. One cyclic peptide, cCPP 15, dramatically outperformed all others, exceeding the benchmark TAT peptide by roughly 40%. This champion sequence combines a trans-4-amino-L-proline modified with a naphth-2-ylmethylene group, positioned at a type II β-turn, with four D-arginine residues split between endocyclic and exocyclic positions. Molecular dynamics simulations showed the naphthalene ring embedding into the lipid bilayer while the arginine guanidinium groups coordinated with membrane phosphate headgroups. Temperature-dependence experiments and inhibitor studies pinpointed dynamin-mediated endocytosis as the dominant uptake route. The peptide accumulated progressively over twelve hours and penetrated six different cell lines, including HeLa, U2OS, and neuroblastoma-derived SH-SY5Y cells, demonstrating broad applicability rather than cell-type specificity. Crucially, when the researchers conjugated an extended version of this scaffold, cCPP 18, to enhanced green fluorescent protein, EGFP, the construct delivered the 27 kDa protein into living cells. Neither EGFP alone nor a simple mixture of EGFP and unconjugated peptide showed any uptake.
The study distills several design rules for future CPP engineering. Secondary amine linkages outperform ethers. Aromatic hydrophobic groups enhance penetration more than aliphatic chains of similar length. Distributing arginine residues around the macrocycle, rather than clustering them, improves membrane interaction. These principles should guide the development of next-generation CPPs capable of shuttling proteins, nucleic acids, or small-molecule drugs past the membrane barrier. The efficiency of cCPP 15 hints at even more ambitious applications, including crossing the blood-brain barrier to reach neurological targets that remain beyond the reach of conventional therapeutics.
Publication Information
Author Information

Yuan Zhang completed his bachelor's and master's degrees in pharmaceutical sciences at Harbin Medical University and Peking Union Medical College, respectively. In 2024, he earned his Ph.D. in Chemical Biology from the University of Copenhagen under the supervision of Professor Morten Meldal. Subsequently, he completed his first postdoctoral training at the same institution, also under the guidance of Professor Meldal. His research involves peptide chemistry, medicinal chemistry, amyloid beta fibrillation inhibition, and cell penetrating peptides. He has published his work in Journal of the American Chemical Society, Angewandte Chemie and other peer reviewed journals.

