Customizable Clicks
Reflecting work in the Yang, Lin, and Guo Labs
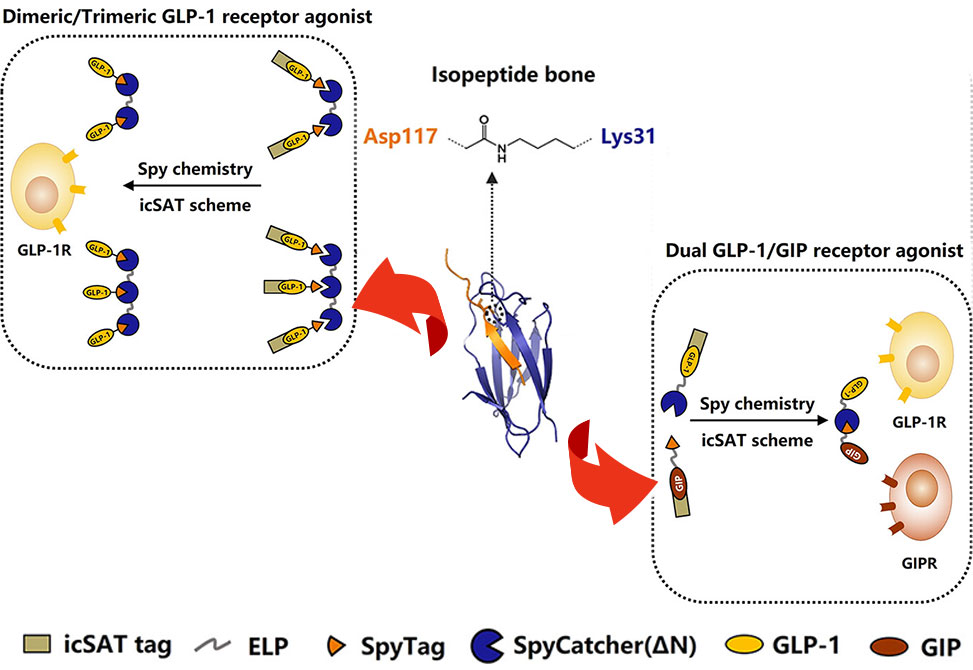
The development of oligomeric glucagon-like peptide-1, GLP-1, and GLP-1-containing coagonists holds promise for enhancing the therapeutic potential of the GLP-1-based drugs for treating type 2 diabetes mellitus, T2DM. In research published in Bioconjugate Chemistry, researchers from the Yang, Lin, and Guo Groups, report a facile, efficient, and customizable strategy based on genetically encoded SpyCatcher-SpyTag chemistry and an inducible, cleavable self-aggregating tag, icSAT, scheme.
icSAT-tagged SpyTag-fused GLP-1 and the dimeric or trimeric SpyCatcher scaffold were designed for dimeric or trimeric GLP-1, while icSAT-tagged SpyCatcher-fused GLP-1 and the icSAT-tagged SpyTag-fused GIP were designed for dual GLP-1/GIP, glucose-dependent insulinotropic polypeptide, receptor agonist. These SpyCatcher- and SpyTag-fused protein pairs were spontaneously ligated directly from the cell lysates. The subsequent icSAT scheme, coupled with a two-step standard column purification, resulted in target proteins with authentic N-termini, with yields ranging from 35 to 65 mg/L and purities exceeding 99%.
In vitro assays revealed 3.0- to 4.1-fold increased activities for dimeric and trimeric GLP-1 compared to mono-GLP-1. The dual GLP-1/GIP receptor agonist exhibited balanced activity toward the GLP-1 receptor or the GIP receptor. All the proteins exhibited 1.8- to 3.0-fold prolonged half-lives in human serum compared to mono-GLP-1 or GIP.
This study provides a generally applicable click biochemistry strategy for developing oligomeric or dual peptide/protein-based drug candidates.