Cytosolic Delivery of Functional Cargoes
Reflecting work in the
The delivery of functional proteins remains a major challenge in advancing biological and pharmaceutical sciences. In research published in JACS, members of the Hackenberger Group at the Humboldt University's Leibniz Research Institute, describe a powerful, simple, and highly effective strategy for the intracellular delivery of functional cargoes.
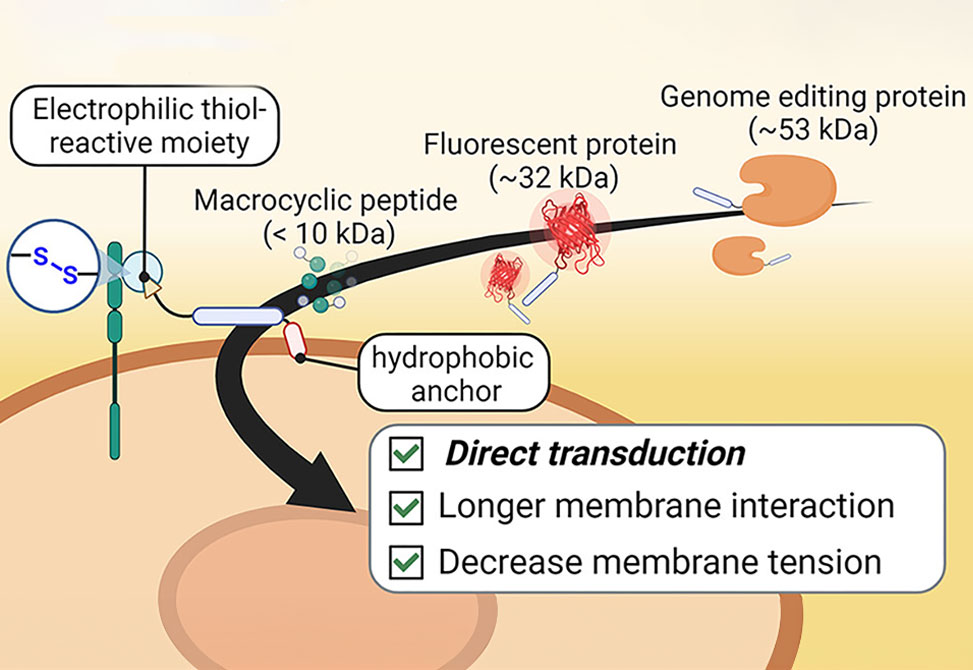
Previously, they have demonstrated that cell-penetrating peptide, CPP, additives equipped with electrophilic thiol-reactive moieties temporarily attach to the cellular membrane, thereby facilitating the cellular uptake of protein- and antibody-CPP cargoes through direct membrane transduction at low concentrations. Now, we hypothesize that CPP-additives with an increased retention on the cellular membrane will further enhance intracellular uptake.
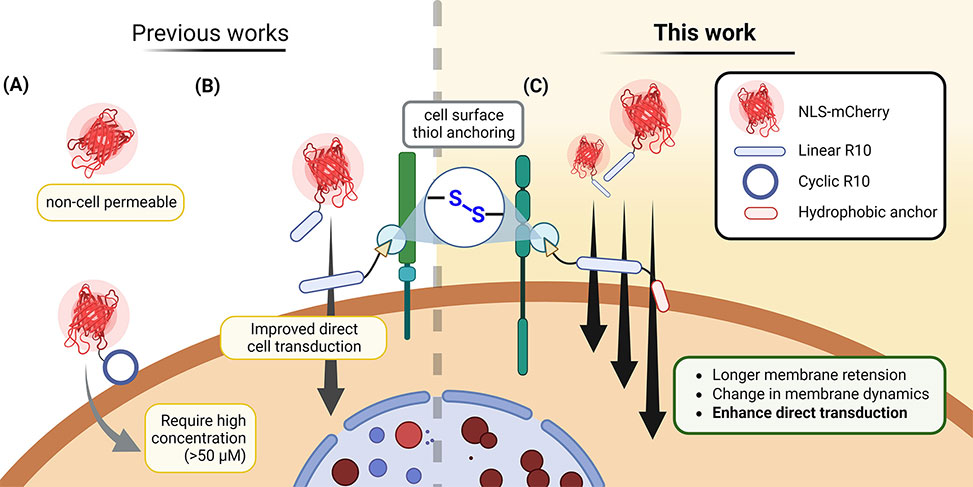
The group members discovered that adding a small hydrophobic peptide sequence to an arginine-rich electrophilic CPP-additive further improved the uptake of protein-CPP conjugates, whereas larger hydrophobic anchors showed increased cytotoxicity. Cell viability and membrane integrity measurements, structure–activity relationship studies, and quantitative evaluation of protein-CPP uptake revealed important design principles for cell-surface-retained CPP-additives. These investigations allowed the group to identify a nontoxic, thiol-reactive CPP-additive containing the hydrophobic ILFF sequence, which can deliver fluorescent model proteins at low micromolar concentrations. This hydrophobic CPP-additive allowed the addition of protein cargoes for intracellular delivery after initial additive incubation. Time-lapse fluorescence microscopy and membrane tension analysis of cells treated with fluorescent ILFF-CPP-additives supported the claim of increased cell surface retention and suggested that the protein-CPP cargoes enter the cell through a mechanism involving lowered cell membrane tension.

Finally, group members demonstrated that their newly engineered hydrophobic CPP-additive enabled the uptake of a functional macrocyclic peptidic MDM2-inhibitor and a recombinant genome editing protein. This indicates that the developed hydrophobic CPP-additive holds promise as a tool to enhance the intracellular delivery of peptide and protein cargoes.