Delivery of Peptide-LYTAC
Reflecting recent work in the Wu, Chen, Zhang, and Luan Groups
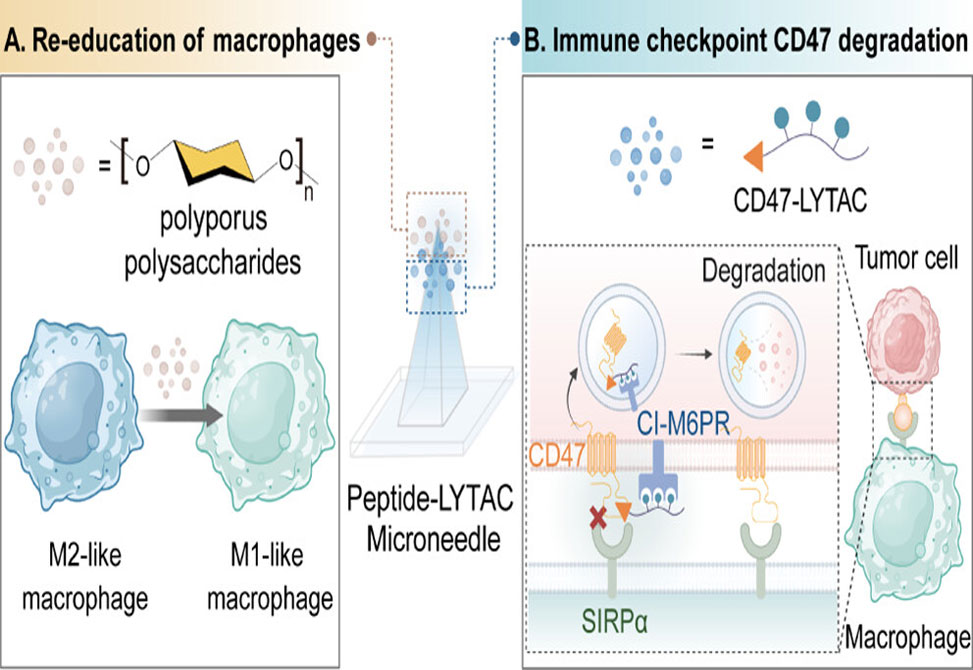
While immune checkpoint blockade, ICB, therapies have transformed cancer immunotherapy, tumors such as melanoma frequently exploit the CD47-SIRPα axis to evade macrophage-mediated clearance. Antibody-based CD47 blockade, though effective, is clinically constrained by off-target toxicity, most notably anemia, due to widespread CD47 expression on red blood cells. To address this, researchers from the Institute of Interdisciplinary Integrative Medicine and the Institute of Medicinal Plant Development in Shanghai, China, report in the Journal of American Chemical Society, the development of a novel peptide-based lysosome-targeting chimera, LYTAC, termed RS17-M6P3, which selectively degrades CD47 in tumor cells via the lysosomal pathway without affecting erythrocytes.
RS17-M6P3 consists of a CD47-binding peptide, RS17, conjugated to a tri-valent mannose-6-phosphate, M6P3, moiety for recruitment of cation-independent M6P receptors, CI-M6PR. This targeted degradation mechanism circumvents the limitations of conventional receptor-occupancy-based therapies. The study confirmed dose- and time-dependent CD47 degradation in B16F10 melanoma and other tumor cell lines, with minimal off-target effects on RBCs due to their lack of lysosomes. Mechanistic studies using fluorescence microscopy and lysosomal inhibitors validated the selective lysosomal trafficking and degradation of CD47.
In macrophage co-culture assays, RS17-M6P3 significantly enhanced phagocytosis of tumor cells compared to CD47-blocking peptides alone, demonstrating superior functional efficacy. Importantly, RS17-M6P3 did not induce hemolysis or macrophage uptake of RBCs, affirming its hematologic safety.
To further potentiate the immune response, the study integrates Polyporus polysaccharide, PPS, a natural immunomodulator that reprograms tumor-associated macrophages, TAMs, from the immunosuppressive M2 phenotype to the tumoricidal M1 phenotype. PPS not only inhibited M2 polarization but also upregulated M1-associated genes and phagocytic activity in both naïve and polarized macrophages. The synergistic combination of RS17-M6P3 and PPS amplified tumor cell phagocytosis in vitro.
This dual-action strategy was encapsulated in a biodegradable microneedle, MN, platform, PPS/RS17-M6P3@MN, enabling localized transdermal delivery. Mechanical and dissolution analyses confirmed optimal performance for dermal penetration and payload release. In murine melanoma models, PPS/RS17-M6P3@MN treatment led to significant tumor growth inhibition, elevated CD4+/CD8+ T-cell infiltration, M1-biased TAM repolarization, and decreased tumor proliferation and viability, without inducing systemic toxicity or hematological abnormalities.
Furthermore, combination therapy with anti-PD-L1 antibodies showed additive tumor regression and improved survival, supporting the compatibility of this LYTAC platform with current checkpoint inhibitors. Intravenous administration of RS17-M6P3 in breast cancer models also demonstrated favorable pharmacodynamic and safety profiles, highlighting its versatility and translational promise.
This is the first demonstration of a peptide-based LYTAC integrated with a natural immunomodulator in a dissolvable MN system, offering a potent, dual-modality therapeutic platform that simultaneously targets immune checkpoints and reconditions the tumor microenvironment. The findings support broader application of LYTAC technologies in safe, selective, and effective cancer immunotherapy.