Dynamic Ion Channels
Reflecting recent work in the the Niitsu Lab
Membrane-spanning peptides that form ion-conductive channels remain a challenging frontier in de novo protein design. While strides have been made in creating water-soluble peptide assemblies, translating that precision to transmembrane systems is significantly more complex due to the dynamic, amphipathic environment of lipid bilayers.
In a groundbreaking international collaborative work led by Dr Ai Niitsu, RIKEN, Japan, Prof. Dek Woolfson, University of Bristol, UK, Prof. Mark Wallace at King’s College London, UK, and also including researchers from the University of Glasgow, the University of Oxford, and the Rajiv Gandhi Centre for Biotechnology, published in the Journal of the American Chemical Society, present a suite of rationally and computationally designed α-helical barrels, αHBs, — modular, membrane-inserting peptides that assemble into channels of defined stoichiometry and conductance. These dynamic constructs offer a powerful new toolkit for synthetic biology and bioengineering, enabling voltage-responsive, tunable ion flow through artificial membranes.
Engineering Functional α-Helical Barrels from Coiled-Coil Principles
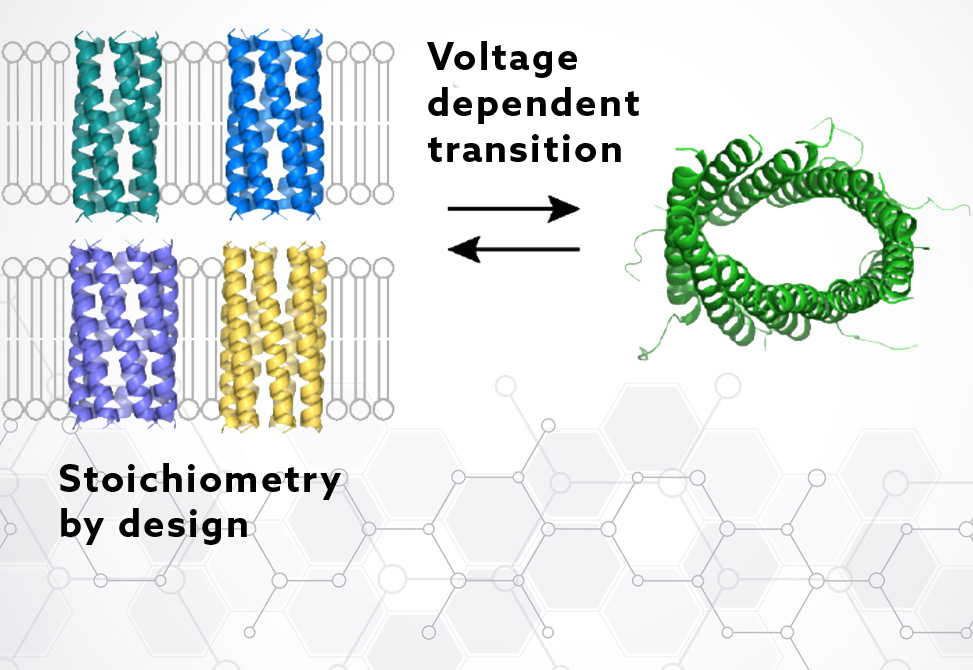
The design centered on coiled-coil packing motifs, leveraging the well-understood knobs-into-holes, KIH, interactions that underlie coiled-coil stability. Using a heptad repeat strategy, g–a–d–e, researchers screened 144 combinations to optimize helix–helix specificity. The lead design, termed CCTM-SaVbIcNd, was predicted to form a membrane-compatible, polar-lined barrel conducive to ion passage.
Peptides were synthesized and experimentally characterized in micellar and bilayer environments. Analytical ultracentrifugation confirmed pentameric assembly, while CD spectroscopy verified high helicity. Atomistic molecular dynamics, MD, simulations revealed a stable, hydrated central pore, supporting its role as a conductive channel.
Two Conductance States: Sequence-Dependent and Dynamic
Electrical recordings in planar lipid bilayers revealed two distinct conductance states for CCTM-SaVbIcNd:
-
Low-Conductance, LC, States: These were stable and sequence-specific, correlating with peptide stoichiometry and lumenal geometry. The LC conductance values varied predictably across sequence variants.
-
High-Conductance, HC, States: These were dynamic and voltage-dependent, marked by discrete stepwise increases in conductance. All peptide variants showed similar HC characteristics, suggesting a shared, stochastic mechanism involving peptide rearrangement or recruitment.
Tuning Geometry Through Rational Substitution
The modular CCTM design proved amenable to rational alterations. Targeted substitutions at the a and c positions in the heptad repeat — which directly impact helix–helix interface angles — successfully shifted stoichiometries:
-
Heptameric barrels were achieved by replacing Ile with Ala (CCTM-SaVbAcNd) or Ser with Thr (CCTM-TaVbIcNd).
-
A hexameric variant (CCTM-SaVbIcNd-S20C-ace) was created via Ser → Cys substitution and alkylation with iodoacetamide, introducing steric bulk at a key interface.
-
These substitutions validated the design logic: smaller side chains permitted tighter packing, favoring heptamers, while bulkier groups expanded the barrel, yielding hexamers or pentamers.
Single-Channel Optical Recordings Confirm Dynamic Behavior
To determine whether HC states arose from single barrels or multiple channels, optical single-channel recordings, oSCR, were performed using droplet interface bilayers. The results were definitive:
-
HC steps originated from individual peptide barrels, not multiple insertions.
-
Channels displayed dynamic, reversible transitions between conductance states, consistent with changes in barrel radius or stoichiometry.
Further MD simulations of decameric and larger barrels under high membrane potentials supported this view, showing stable, elliptically shaped pores that collapsed in the absence of voltage — reinforcing the notion of voltage-gated structural plasticity.
Sequence–Stoichiometry–Function Relationships: Toward Predictive Design
While high-resolution structural data were not available, the study revealed strong correlations between sequence variants, oligomeric stoichiometry, and resulting conductance profiles:
-
Tightly packed heptamers with Ala-rich interiors yielded the lowest conductance.
-
Looser, polar-lined pentamers supported higher ion flow.
-
Exterior-facing mutations — for example, Trp to Lys — had minimal effect, suggesting that lumenal residues primarily dictate channel behavior.
These results support a rational design approach based on sequence-driven modulation of geometry and function — laying the groundwork for predictive engineering of synthetic ion channels, even in the absence of atomic-level structural data.
Conclusion: Toward Designer Membrane Channels with Precision Control
This work demonstrates a rational path to constructing de novo membrane-spanning ion channels with programmable architecture and conductance. The duality of stable low-conductance states and dynamic, voltage-dependent expansion mirrors natural mechanisms seen in antimicrobial peptides like alamethicin — but with unprecedented control over sequence and structure.
These insights pave the way for next-generation applications, including:
-
Voltage-sensitive biosensors
-
Selective filtration systems
-
Synthetic cells with tunable permeability
-
Engineered microbes for bioproduction
The study underscores a critical principle in synthetic biology: with the right design rules, we can not only mimic biological functions — we can reimagine them.