Electrophilic Warheads
Reflecting work in the Raj Lab

Key Findings
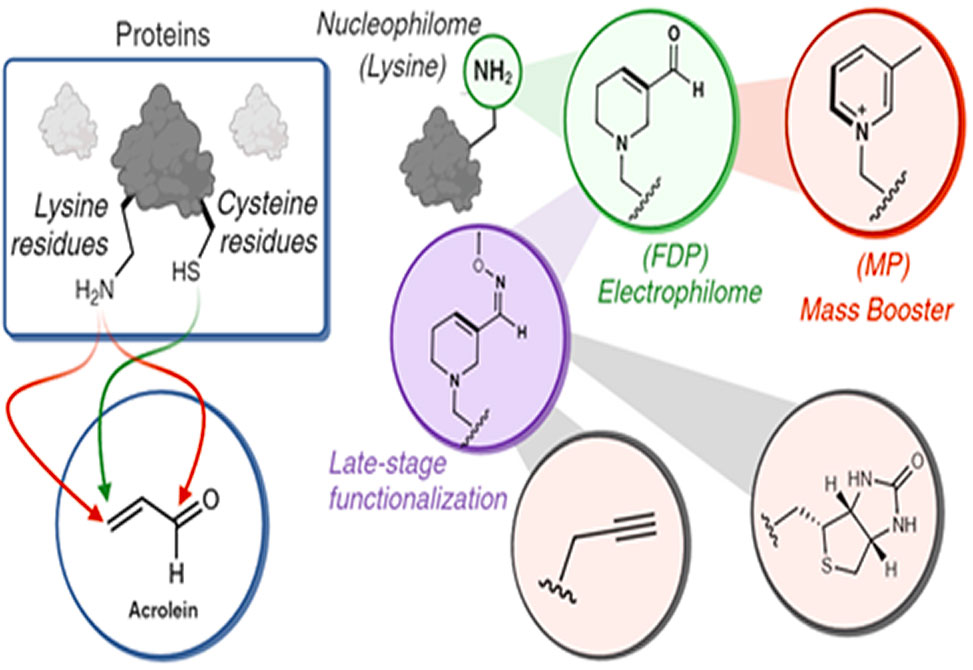
Selective Lysine Modification: FDP-lysine undergoes a secondary transformation into another heterocycle, 3-methylpyridinium, MP, lysine, through reagentless deoxygenation and aromatization.
Protein Engineering and Functionalization: FDP-modified lysines serve as reactive handles for conjugation with diverse payloads, enabling efficient peptide and protein modification.
Chemoproteomic Profiling: A systematic analysis of FDP- and MP-modified proteins uncovered approximately 1,548 novel cross-linking partners, highlighting acrolein-induced changes in protein–protein interactions.

The Monika Raj Lab at Emory University
Advancing Protein Modification Techniques
Current methods for detecting and characterizing protein modifications—such as mass spectrometry, SDS-PAGE, and antibody-based detection—are limited in their ability to identify site-specific changes and cross-linking events. This new approach directly addresses these challenges by:
Identifying site-specific protein modifications in biological environments.
Characterizing the chemical nature of these modifications.
Pinpointing precise modification sites.
Determining cross-linking interactions between proteins.
Mechanism of the Reaction
The transformation of lysine into FDP occurs through a stepwise reaction involving two Michael additions to acrolein, followed by an aldol reaction and dehydration. The resulting electrophilic FDP moiety than converts to MP-lysine upon gentle heating, with its mass-boosting properties making it a powerful tool for tracking protein modifications.
Versatility in Protein Functionalization:
FDP-lysine enables the attachment of functional groups, facilitating late-stage peptide modification and proteome profiling.
Selective Protein Labeling:
This method allows for homogeneous modification of proteins with high specificity, offering new insights into acrolein-mediated protein interactions.
Impact and Applications
Drug Discovery:
This strategy enhances peptide and protein engineering for targeted drug development.
Toxicology and Disease Research:
The ability to track acrolein-modified proteins offers new insights into the molecular mechanisms underlying conditions such as neurodegenerative diseases, cardiovascular disorders, and cancer.
Proteomics and Biomarker Identification:
The technique provides a powerful platform for uncovering novel protein biomarkers and metabolite-mediated interactions.
Conclusion
This study presents a novel and highly efficient chemoselective reaction that converts lysine into an electrophilic warhead, revolutionizing protein modification methodologies. By leveraging this chemistry, researchers can explore new dimensions in protein engineering, uncover previously unknown protein interactions, and advance the development of biomarker-driven diagnostics and therapeutics.