Engineered Lasso Peptides
Reflecting work in the Lassogen Lab
A major barrier to durable responses with PD-1/PD-L1 checkpoint inhibitors is the activation of immunosuppressive TGF-β within the tumor microenvironment. This immunosuppression is driven in large part by the RGD-binding integrins αvβ6 and αvβ8, which are upregulated across many solid tumors. Direct systemic blockade of TGF-β has proven toxic, and broad integrin inhibition risks off-target liabilities,such as bleeding events via αIIbβ3 inhibition. Conventional peptide scaffolds also struggle with in vivo stability and manufacturability.
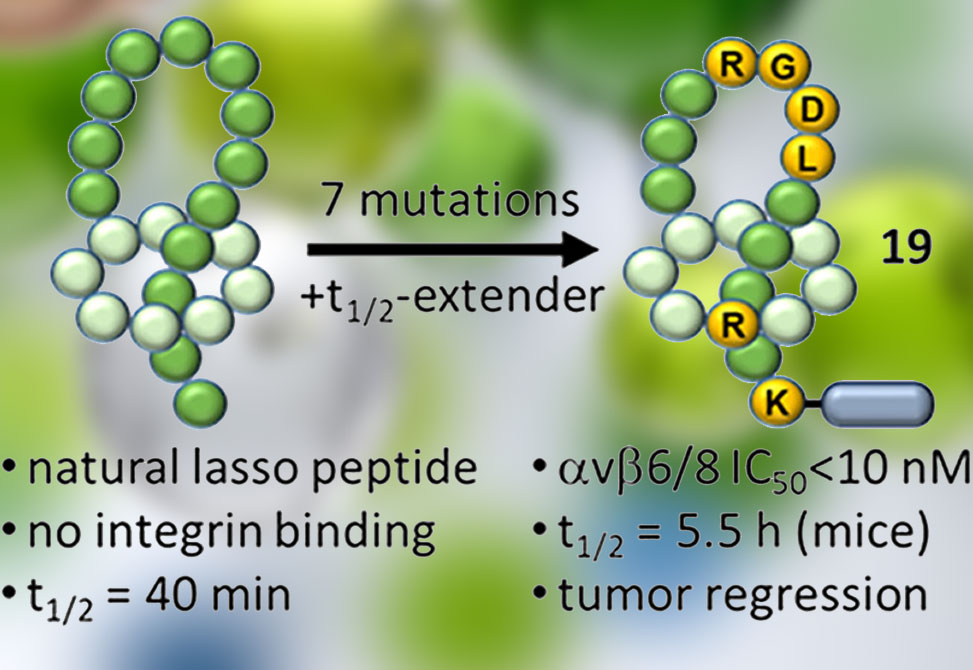
To address these challenges, researchers from San Diego-based Lassogen, whose mission is to unlock the therapeutic potential of engineered Lassotides™, reports in JACS the use of lasso peptides with a rigid loop-over-ring topology as a scaffold for dual, selective inhibition of αvβ6 and αvβ8. Starting from the naturally occurring lasso peptide microcin J25, the team grafted the RGDL epitope and iteratively optimized via epitope scanning, structure-guided modeling, and directed evolution of loop, ring, and tail residues. Combining ring substitutions, A3R/T, with I16Q and additional loop/ring edits yielded lassotides with low-nanomolar to sub-nanomolar potency against both integrins, for example, lassotide analogs 12–18 afforded IC50 values of ~0.7–3 nM. Multi-D NMR confirmed how the scaffolds rigidly presents RGDL in the loop. Computational models predicted that modified ring residues could contribute ancillary β-subunit contacts and allowed further refinement of the binding geometry.
Translational optimization focused on half-life extension without sacrificing potency. Site-specific installation of a PEG2–p-iodophenylbutyrate albumin binder at K20 or K3 produced conjugates such as lassotide 19 that retained dual-integrin potency and sharpened selectivity across the RGD family. Lassotide 19 inhibited αvβ6/αvβ8 at single-digit nanomolar IC50 while maintaining >150-fold selectivity over other RGD integrins, including minimal activity on αIIbβ3. Functional assays mirrored this profile. In MLEC luciferase readouts, 19 blocked αvβ6/8-mediated TGF-β activation to levels comparable with small-molecule standards.
Manufacturability, a historical obstacle for lasso peptides, was overcome through host engineering, pathway optimization, and cyclase redesign. For example, when using the engineered cyclase McjC K252A/K388A, titers climbed from tens of mg/L in flasks to multi-gram per liter yields in fed-batch fermentation. Lassotide 10 reached ~5.65 g/L in 5-L reactors with uniform performance across tanks, enabling >80 g isolation from 20 L broth. This represents the highest reported lasso yield to date and removes a central development bottleneck.
Drug-like PK tracked directly with albumin binding. Compared with the short-lived parent 11 with mouse t½ ~0.6 h, lassotide 19 achieved ~9-fold longer half-life in mice, t½ ~5.5 h, and 8.5 h in rats, with >25-fold AUC gains and >99% plasma protein binding. Double acylation, 24, extended mouse t½ to ~20 h, demonstrating tunable exposure. Tissue distribution placed high concentrations in ovary, mammary, lung, and kidney—sites relevant to αvβ6/8-positive tumors—while sparing platelet targets. Safety screens across 47 liability assays were clean to ~40–45 μM, and both plasma and hepatocyte stability were favorable.
Efficacy emerged where predicted. In the anti-PD-1–resistant EMT6 TNBC model, anti-PD-1 alone or with the short-lived parent 11 had minimal effect, but 19 + anti-PD-1 halted tumor growth and induced multiple regressions, with clear dose-response and durable immunity upon rechallenge. In the ID8 ovarian cancer model, the same combination yielded regression in 5/7 tumors, 2 complete responses, and significant survival benefit that persisted post-treatment—consistent with relief of stromal TGF-β suppression. Across integrin profiling, conjugates preserved the desired narrow spectrum: potent αvβ6/8 inhibition with minimal off-target activity.
This study demonstrates that lasso peptides can be precisely engineered, manufactured at scale, and tuned for clinically relevant PK while maintaining exceptional dual selectivity for αvβ6/8. Conceptually, the work highlights 1| the loop-ring orthogonality of the lasso scaffold as a modular handle for separating binding from PK chemistry, 2| selective αvβ6/8 blockade as a tumor-focused alternative to systemic TGF-β inhibition, and 3| the readiness of this modality for translational exploration. The advance is clear: lasso peptides are no longer exotic curiosities but practical immuno-oncology therapeutics, with a direct path to resensitizing checkpoint inhibitor-refractory tumors. Moreover, this work provides a blueprint for further advances using this untapped modality in oncology and beyond.