Ephemeral Pores
Reflecting work in the Cotten and Pastor Labs
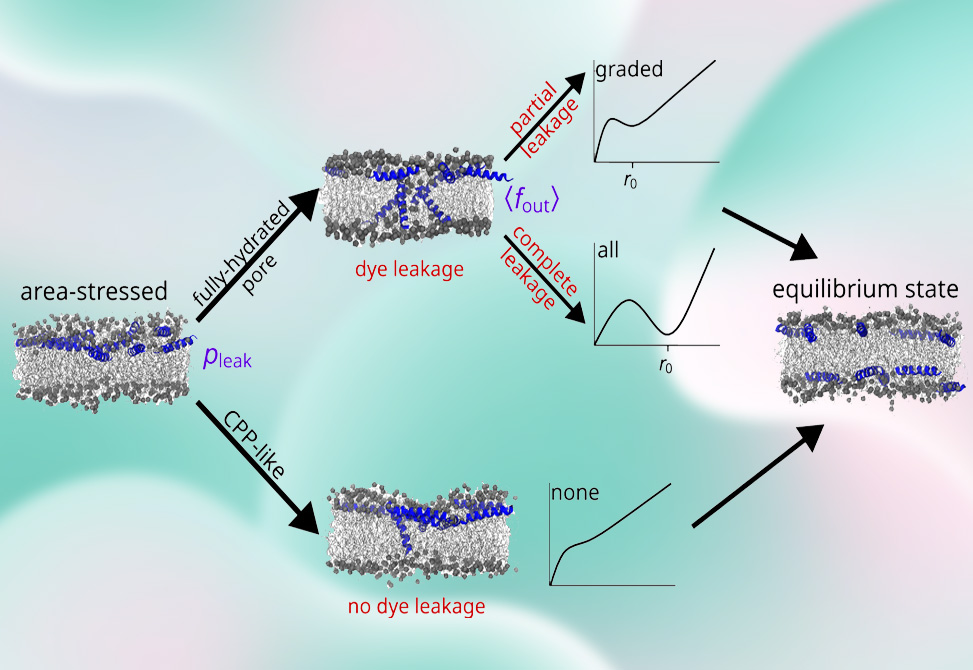
Membrane-active peptides, MAPs, span two archetypes: antimicrobial peptides, AMPs, which transiently porate membranes and kill cells, and cell-penetrating peptides, CPPs, which cross bilayers with little overt permeabilization to deliver cargo intracellularly. Despite decades of study, the structural basis of their hallmark transient leakage—rapid dye efflux that self-terminates and can be reinitiated by adding more peptide—has remained elusive. The central obstacle is that leakage depends not only on peptide sequence and dose but also on bilayer composition, curvature stresses, and kinetic pathways that are inherently nonequilibrium. In a PNAS study, researchers from the Pastor group at the National Institutes of Health, NIH, and the Cotten group at Oregon State University present a combined experimental/simulation framework using the AMP piscidin-1, P1 that establishes a single quantitative mechanism linking graded and all-or-none release via asymmetry-driven poration.
When cationic amphipathic peptides first bind the outer leaflet of a vesicle, they create leaflet area asymmetry and surface-pressure imbalance. This “area stress” lowers the free-energy cost of forming transient pores. Two poration pathways relieve the stress: 1| CPP-like pores—narrow, short-lived defects that permit lipid and peptide flip-flop with negligible solute loss, “none” in all-or-none, and 2| fully hydrated metastable pores—larger defects that rapidly leak dye before closing. The observed leakage mode is not binary, it reflects the competition between these pathways, governed by lipid composition, local peptide concentration, and the evolving free-energy landscape as asymmetry is dissipated.
Start–stop dye-release assays on LUVs show that P1 causes a burst of leakage that levels off within ~1 h and restarts upon re-addition, consistent with a self-limiting, stress-relaxation process. Crucially, lipid composition alone toggles the apparent mode: in 3:1 POPC:POPG, P1 yields graded leakage; adding lysophosphatidylcholine to make 2:1:1 POPC:POPG:lysoPC shifts behavior to predominantly all-or-none. Kinetically, lysoPC accelerates efflux: at L/P ≈ 60, the first-burst rate is ~3.3-fold higher, ≈2.7×10-1 vs 8.1×10-2 min-1. Fluorescence requenching curves capture these regimes: monotonic increases in the graded system, and flat or nonmonotonic responses in lysoPC membranes indicative of all-or-none. These data argue that packing defects and positive spontaneous curvature, introduced by lysoPC, favor formation and persistence of larger pores.
Molecular dynamics with potentials of mean force, PMFs, compare area-stressed, AS, bilayers with all peptides on one leaflet, for example, 10/0, ten on one leaflet, none in the other, to area-relaxed, AR, with peptides symmetrically distributed as in 5/5, five in each leaflet. Area stress dramatically reduces the barriers for water-wire initiation, CPP-like pore formation, and pore expansion. For example, in AS 10/0 membranes the free energy to form a 10 Å radius pore is ~12.4 kcal·mol-1, versus ~22.7 kcal·mol-1 in AR 5/5, an ~10.3 kcal·mol-1 stabilization that explains rapid, stress-driven poration. LysoPC further lowers barriers: water-wire, CPP-like, and 10 Å pore formation are each ~2–8 kcal·mol-1 easier than in lysoPC-free POPC:POPG at the same peptide asymmetry. Only AS systems exhibit plateaus/local minima in the PMF at small radii, consistent with metastable pores; AR systems show no such minima, and pores close swiftly.
Unbiased simulations starting from pre-formed pores confirm these energetics. In AS bilayers, fully hydrated pores often persist for microseconds, accompanied by rapid lipid translocation, ≈10–12 net flips within hundreds of nanoseconds, and occasional peptide translocation, both hallmarks of asymmetry relaxation. In AR membranes, the same pores collapse within tens to hundreds of nanoseconds with negligible lipid movement. Mechanistically, peptides in AS systems tilt and insert more deeply, termini crossing the midplane, as pores nucleate and grow, reducing overcrowding in the outer leaflet and stabilizing the defect; this deep insertion is rare in AR controls.
Because peptide distributions are inhomogeneous on vesicle surfaces, local microdomains experience different effective concentrations and defect propensities. The authors formalize this by allowing two functions of peptide: 1| the probability that a vesicle leaks, reflecting the fraction of domains that enter the hydrated-pore pathway, and 2| the average fractional release from leaking vesicles, reflecting pore size/lifetime. With these two sigmoidal controls, a single model recapitulates ideal graded, increasing requenching signal, ideal all-or-none, flat, and nonmonotonic mixed behavior, rising at low dose, flattening/decreasing at higher dose. Fitted to the P1 data, the unified model yields better goodness-of-fit than classical graded or all-or-none models while remaining mechanistically interpretable in terms of pore energetics and domain heterogeneity.
The framework elevates membrane context to a first-class design parameter: lipids that introduce positive curvature and packing defects, for example, lysoPC, lower line tension and raise pore lifetimes/radii, biasing toward all-or-none. Conversely, compositions with fewer defects and more negative curvature bias toward small, rapidly closing pores and graded release. Peptide variables—charge, amphipathicity, hydrophobic moment, and insertion propensity—shift the same landscape. Practically, one can 1| tune selectivity by matching sequence features to the defect spectrum of target membranes, 2| exploit stapling/oligomerization tendencies that raise local surface concentration and favor hydrated pores in desired contexts, and 3| integrate requenching kinetics with potency/cytotoxicity readouts to map sequence–mechanism–activity relationships. Because the model is quantitative and rooted in PMFs, it provides a clear path to in silico screening or machine-learning surrogates informed by measurable parameters, EC50, burst rates, requenching curves.
Transient poration by MAPs emerges from a single, asymmetry-driven mechanism in which area stress lowers pore-formation barriers, two pore classes compete to relax stress, and leakage terminates once asymmetry dissipates. Lipid composition, defects, curvature, peptide distribution, local crowding, and sequence-encoded insertion dynamics jointly set pore probability, size, and lifetime, thereby spanning the full continuum from graded to all-or-none release. This unified view turns long-standing phenomenology into predictive design rules for AMPs and CPPs, enabling rational targeting of membranes with enhanced cellular specificity.
Publication Information
Author Information

Dr. Amy Rice received her Ph.D. in Physics from the Illinois Institute of Technology in 2019. She joined the Laboratory of Membrane Biophysics at the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, first as a Postdoctoral Fellow in 2019 and has served as a Research Fellow since 2024. In 2025, she was recognized as Outstanding Fellow of the Year by the NHLBI. Dr. Rice has presented her research at numerous venues, including the Biophysical Society Annual Meeting and the CHARMM Developers Meeting, and actively mentors undergraduate and graduate researchers. She is also a member of the NHLBI Fellows Advisory Committee and the American Institute of Physics’ Adopt-A-Physicist program. Her research employs molecular dynamics simulations to probe the structure and dynamics of cell membranes, focusing on interactions between model bilayers and membrane-active peptides to elucidate the mechanisms of peptide-induced poration.

