Ephrin Receptors
Reflecting work in the Sawyer Lab
Ephrin, Eph, receptors are the largest family of receptor tyrosine kinases. Interactions between Eph receptors and their membrane-bound ephrin protein ligands are associated with many developmental processes as well as various cancers and neurodegenerative diseases. With significant crosstalk between different Eph receptors and ephrin ligands, there is an urgent need for high-affinity ligands that bind specifically to individual Eph receptors to interrogate and modulate their functions.

From left to right, Sophie Epstein, Nicholas Sawyer, and Jessica Tennett. Both Sophie and Jessica were undergraduate researchers in the Sawyer Group. Jessica graduated in 2023 and is currently a graduate student at the University of Oxford. Sophie graduated in May of 2024, and is currently a clinical research assistant at Mount Sinai Hospital.
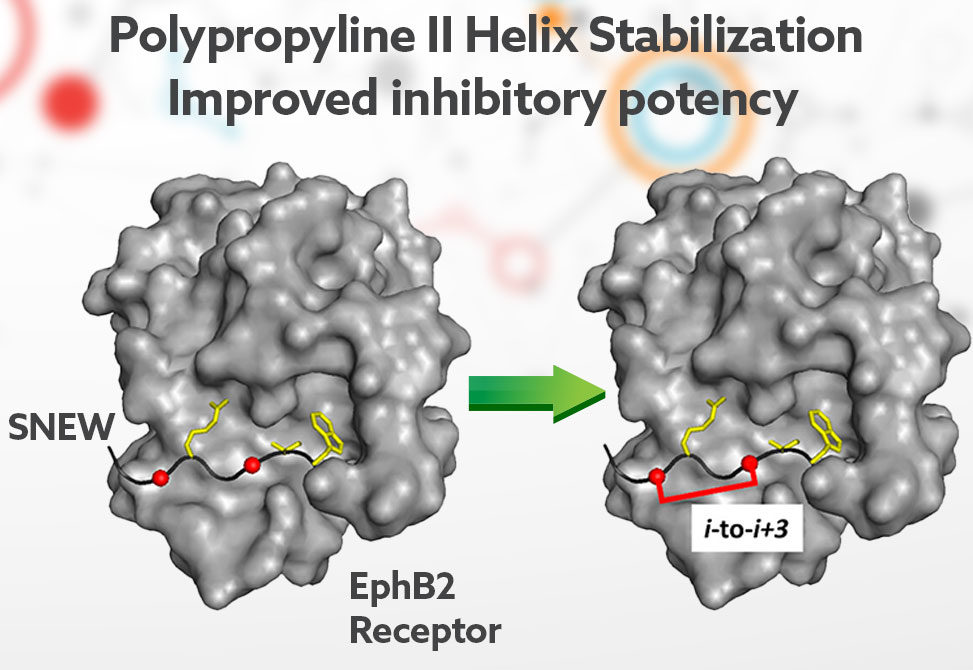
In work published in ACS Chemical Biology, researchers in the Nicholas Sawyer lab at Fordham University, describe the rational development of potent EphB2 receptor inhibitors derived from the EphB2 receptor-specific SNEW peptide. To improve inhibitory potency, they evaluated 20+ cross-linkers with the goal of spanning and stabilizing a single polyproline II helical turn observed when SNEW binds to the EphB2 receptor.
Of the cross-linkers evaluated, an 11-atom cross-linker, composed of a rigid 2,7-dimethylnaphthyl moiety between two cysteine residues, was found to yield the most potent inhibitor. Analysis of the cyclized region of this peptide by NMR and molecular dynamics simulations suggests that cross-linking stabilizes the receptor-bound polyproline II helix structure observed in the receptor–peptide complex. Cross-linked SNEW variants retained binding specificity for EphB2 and showed cross-linker-dependent resistance to trypsin proteolysis.
Beyond the discovery of more potent EphB2 receptor inhibitors, these studies illustrate a novel cyclization approach with potential to stabilize polyproline II helical structure in various peptides for specific targeting of the myriad protein–protein interactions, PPIs, mediated by polyproline II helices.