Published in the Journal of the American Chemical Society, researchers in the lab of Dr. Joel P. Schneider at NIH, have unveiled a groundbreaking molecular design strategy that shifts gel-forming peptides into the crystalline state, revealing, for the first time, a high-resolution structure of a parallel rippled β-sheet — a long-theorized but previously unobserved motif predicted by Pauling and Corey over 70 years ago.
Self-assembling peptide hydrogels are central to biomaterials development, offering promising applications in drug delivery, tissue engineering, and immunomodulation. While racemic mixtures of L- and D-peptides have shown advantageous material properties — including enhanced stiffness, protease resistance, and thermal stability — the field has lacked atomic-level insights into the supramolecular interactions that govern these effects.
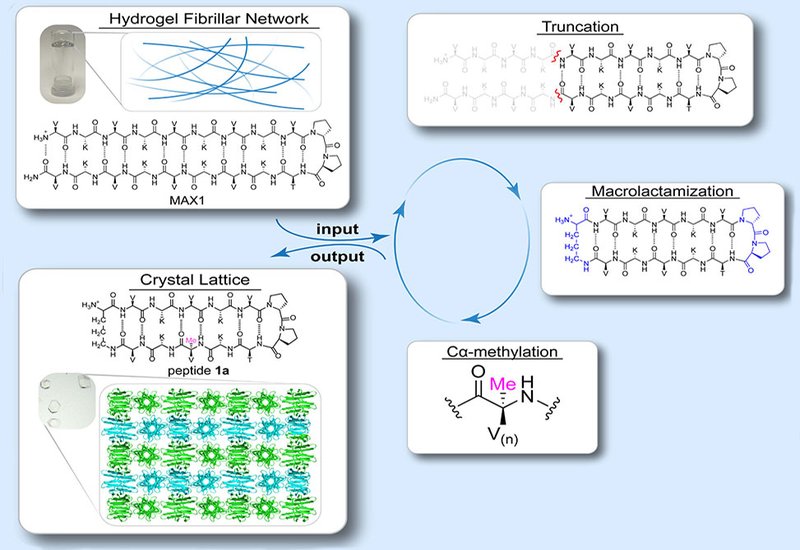
To overcome the crystallization challenges posed by flexible, fibrillar gel networks, the team developed a deliberate structure-guided approach: they truncated the parent MAX1 peptide, introduced cyclization via δ-linked ornithine, and incorporated Cα-methylation to guide and restrict hydrogen bonding. These modifications transformed the assembly behavior, yielding mirror-image macrocyclic peptides capable of forming crystals instead of gels.
High-resolution X-ray crystallography revealed that the racemic mixture of these cyclic peptides self-assembles into flat, fibril-like structures composed of alternating blocks of enantiopure β-hairpins. These domains form both canonical pleated β-sheets and — at the heterochiral interface — a parallel rippled β-sheet stabilized by interdigitated side chains and complementary van der Waals interactions. This ripple structure is framed by flanking Cα-methyl groups that define its registry and boundary — an elegant molecular bookend.
Importantly, these racemic fibrils were denser and more tightly packed than their homochiral counterparts, offering a plausible structural basis for the enhanced mechanical properties observed in racemic hydrogels. The findings not only validate long-standing theoretical predictions but also open a design pathway to engineer stereocomplexed hydrogels with atomic precision.
This work demonstrates that phase-state modulation through rational molecular design can serve as a powerful tool for revealing fundamental structural motifs. The identification of parallel rippled β-sheets provides a new scaffold for future biomaterials and invites the design of responsive, enantioselective peptide systems with built-in control over morphology, mechanics, and function.