Genetical Encoding
Reflecting work in the Schultz Lab
Nicotinamide-containing cofactors play an essential role in many enzymes that catalyze two-electron redox reactions. However, it is difficult to engineer nicotinamide binding sites into proteins due to the extended nature of the cofactor–protein interface and the precise orientation of the nicotinamide moiety required for efficient electron transfer to or from the substrate.

The Schultz Group at Scripps at La Jolla, CA
To address these challenges, researchers at the Peter Schultz lab at Scripps, La Jolla, published in Biochemsitry, genetically encoded a noncanonical amino acid, ncAA, bearing a nicotinamide side chain in bacteria.
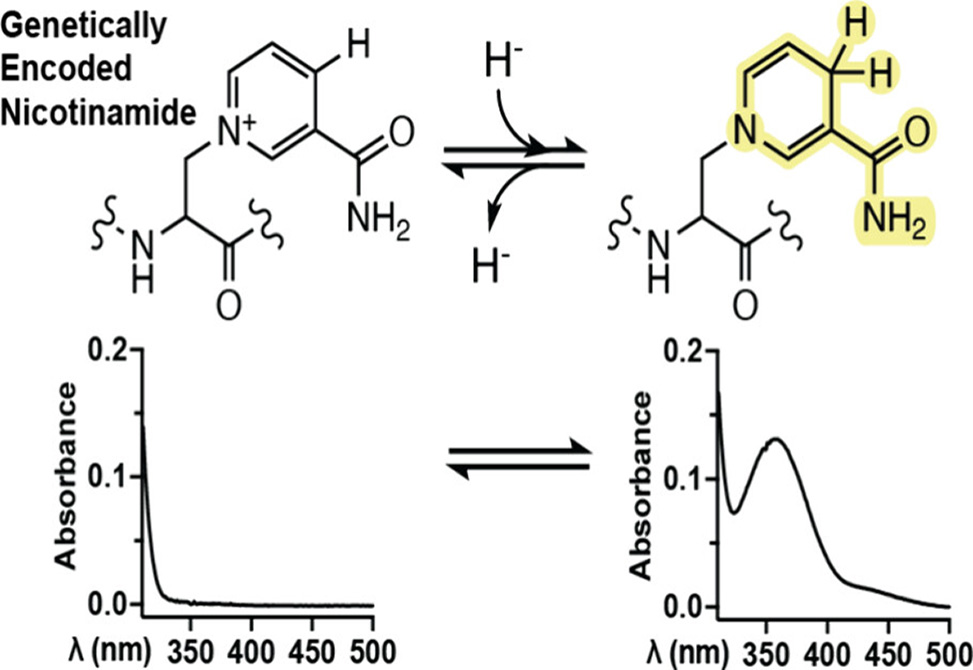
This redox-active amino acid, termed Nic1, exhibits similar electrochemical properties to the natural cofactor nicotinamide adenine dinucleotide (NAD+). Nic1 can be reversibly reduced and oxidized using chemical reagents both free in solution and when incorporated into a model protein.
This genetically encodable cofactor can be introduced into proteins in a site-specific fashion and may serve as a tool to study electron-transfer mechanisms in enzymes and to engineer redox-active proteins.