Glycan–Metal Synergy
Reflecting work in the Koksch Lab
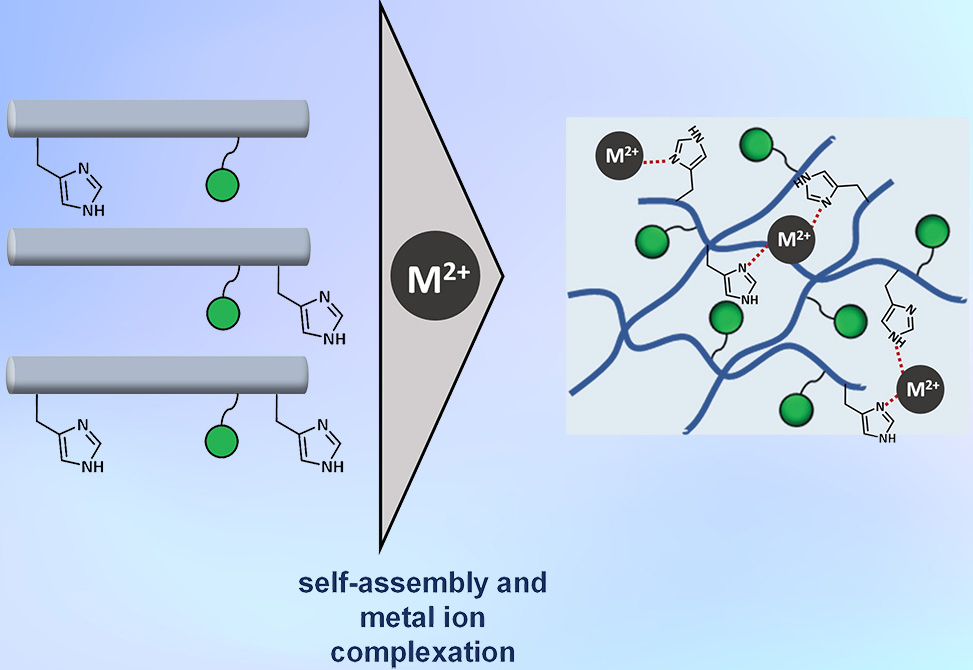
Hydrogels, highly hydrated polymer networks, underpin both natural barriers such as mucus and numerous biomedical applications. Mucins, the glycoprotein constituents of mucus, achieve their viscoelasticity through peptide backbones enriched in proline, threonine, and serine interlaced with complex glycans. Mimicking this dual peptide-glycan architecture has proven difficult, yet essential for probing mucus functions in health and disease. Recent strategies have employed the α-helical coiled-coil scaffold hFF03 as a modular hydrogelator. Researchers in the Koksch Group at the Freie Universität Berlin, published in Peptide Science, systematically introduce histidine residues and monosaccharides into hFF03 to evaluate how glycosylation and divalent metal coordination shape peptide structure, rheology, and morphology.
The 26-residue hFF03 scaffold was diversified by substituting Lys at position 4, 25, or both with His, thereby creating accessible imidazole ligands for metal binding. In parallel, mannose or galactose was conjugated at Lys18 via a glutaric anhydride linker. The resulting library encompassed twelve (glyco)peptides, including His-free controls. Circular dichroism, CD, oscillatory rheology, and cryo-transmission electron microscopy, cryo-TEM, were employed to assess conformational and viscoelastic consequences of Cu2+, Zn2+, Fe2+, or Ca2+ addition under physiological pH and temperature.

CD analysis revealed that metal-free glycopeptides largely preserved higher-order structures characteristic of hFF03. However, incorporation of His introduced metal sensitivity. Position was decisive: His at residue 4 maintained robust higher-order structure but adopted more α-helical character upon Cu2+ addition. His at residue 25 yielded atypical CD signatures, including non-assignable spectra with Zn2+ or Ca2+, suggesting local distortion or C-terminal imidazole–carboxylate interactions. Double substitution, K4H/K25H, produced pH-sensitive peptides prone to precipitation above pH 7.4, yet demonstrated marked metal-dependent conformational switching. Glycan identity further modulated these behaviors, with galactose often favoring helical stabilization compared to mannose.
Viscoelastic profiling uncovered a delicate interplay between histidine placement, glycan identity, and metal coordination. His at position 4: hydrogels retained stiffness comparable to hFF03; mannosylation softened gels, while galactosylation stiffened them. Cu2+ generally reduced storage modulus, G′, though Cu2+/Gal combinations could increase rigidity. His at position 25: substitution alone markedly weakened hydrogels, ~80% reduction in G′. Yet, glycosylation reversed this trend: hFF-K25H-GA-Gal displayed the highest stiffness across the library, particularly with Cu2+, highlighting cooperative influence from glycan hydroxyl coordination. Double His substitution: despite expectations of cumulative coordination, K4H/K25H variants did not universally stiffen gels; effects were glycan- and metal-specific. Notably, glycosylated disubstituted peptides showed net stiffening upon metal addition, whereas aglycons softened.
Cryo-TEM underscored these rheological findings. hFF-K25H-GA-Gal formed long, slender fibrils in the absence of metal but collapsed into dense fiber bundles upon Cu2+ addition. Dark electron-dense spots along the bundles suggested localized Cu2+ clusters cross-linking individual fibrils, consistent with heightened viscoelasticity. By contrast, His4 variants displayed no morphological response to copper, affirming residue position as a key determinant.
Hydrogel mechanics can be tuned with remarkable sensitivity by triadic interplay among histidine location, glycan identity, and divalent metal ions. While His25 alone compromises gel strength, His25 coupled with galactose and Cu2+ yields the stiffest hydrogels, mimicking the responsiveness of native mucins to trace metal concentrations. These findings establish design rules for biomimetic glycopeptide hydrogels and highlight the potential of combining site-specific glycosylation with pH-switchable histidine coordination to engineer adaptive, stimulus-responsive materials.
Publication Information
Author Information

Annelie Puhlmann is a second-year PhD candidate in the laboratory of Professor Beate Koksch at the Department of Chemistry of the Freie Universität Berlin. She obtained her Bachelor’s and Master’s of Science in chemistry, and her current research focuses on peptides and glycopeptides.
Peptides and proteins, as well as their glycosylated versions, are nature’s workhorses with properties ranging from catalysis to structural roles and maintaining the health and homeostasis of complex organisms. The biological context most closely related to Annelie’s work is the human mucosal environment, and in particular the very high moleculer weight and highly glycosylated proteins referred to as mucins.
Using the design tools and synthetic approaches that chemistry offers to develop simpler and chemically precisely defined model systems, Annelie has explored how glycosylation and metal complexation influence structural characteristics and viscoelastic properties of related peptides that spontaneousy form hydrogels.
Currently she is focusing increasingly on alternative strategies for cross-linking in order to provide for more desirable viscoelastic profiles. As a member of the CRC 1449, the aim of her work is to gain an understanding about the influence of peptide composition and glycosylation and how these aspects may work to enhance or diminish the impacts of other judicious chemical modifications.

