Protein–protein interactions, PPIs, are fundamental to cellular regulation, yet targeting them therapeutically remains challenging due to their extensive, shallow interfaces. Peptides offer an attractive solution, providing structural fidelity to natural binding partners while offering tunability in affinity, selectivity, and physicochemical properties. This collaborative study, published in ACS Chemical Biology, by researchers from the Andrew Wilson and Dek Woolfson groups at the Schools of Chemistry at the University of Birmingham and Bristol, respectively, and the Laura Itzhaki group from the Department of Pharmacology at the University of Cambridge, present a sophisticated strategy leveraging self-assembled coiled-coil peptide scaffolds to inhibit the antiapoptotic protein MCL-1, a clinically validated target in cancer.
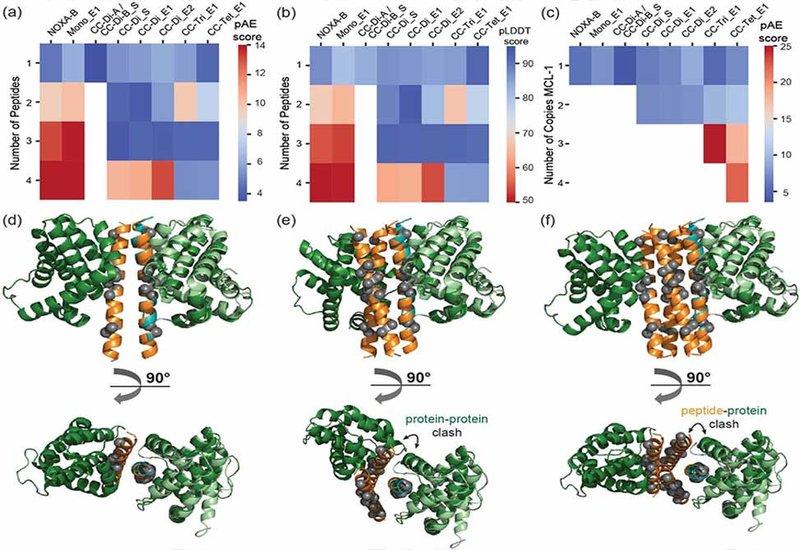
By grafting critical hot-spot residues from the natural MCL-1 binding partner NOXA-B onto the solvent-exposed surfaces of coiled-coil peptides, the researchers constructed a series of homo- and heterodimeric, trimeric, tetrameric, and monomeric scaffolds. These designs allow systematic exploration of how oligomerization, multivalency, and cooperativity influence PPI inhibition. Biophysical characterization, including AlphaFold2 modeling, fluorescence anisotropy, circular dichroism, CD, spectroscopy, native mass spectrometry, MS, size-exclusion chromatography, SEC, and analytical ultracentrifugation, AUC, revealed that the homodimeric coiled-coil scaffold offers the optimal balance between stability, target engagement, and inhibitory potency.
Binding studies demonstrated that homodimeric coiled coils engage MCL-1 with mid-nanomolar inhibitory potency and exhibit positive cooperativity, forming 2:2 peptide:protein complexes that stabilize both partners. This cooperative behavior not only enhances target binding but also increases the thermal stability of the peptide scaffold itself, as evidenced by significant shifts in melting temperatures upon complex formation. The dimeric coiled coils are capable of recruiting two copies of MCL-1, a property not observed in monomeric or higher-order oligomeric designs.
In contrast, trimeric and tetrameric scaffolds displayed reduced potency, a result attributed to steric hindrance that limits the accessibility of grafted binding motifs. Structural modeling supports this observation, showing that excessive oligomerization leads to binding-site occlusion, preventing full engagement of MCL-1. Native MS and AUC corroborate that these higher-order scaffolds fail to maintain stable complexes with multiple MCL-1 molecules, and in some cases, oligomerization competes directly with target binding.
Interestingly, a monomeric control peptide—lacking a stable coiled-coil core but preserving the hot-spot motif—also achieved potent inhibition, highlighting the role of bind-and-fold mechanisms characteristic of MCL-1 interactions. However, this monomeric form does not benefit from the cooperative stabilization observed in the dimeric scaffold.
This study establishes the dimeric coiled-coil architecture as an exceptionally effective platform for designing inhibitors of α-helix-mediated PPIs. The capacity for cooperative binding and dual-target recruitment positions these scaffolds as promising candidates not only for direct PPI inhibition but also for the design of molecular glues capable of assembling ternary complexes for therapeutic applications, such as targeted protein degradation.
The findings further underscore a crucial design principle in peptide-based drug development: multivalency enhances potency only up to the point where structural crowding begins to outweigh the benefits of additional binding interfaces. As such, the dimeric coiled-coil emerges as a Goldilocks solution—sufficiently multivalent to engage targets cooperatively, yet simple enough to avoid self-occlusion.
In conclusion, this work advances the understanding of how self-assembled peptide scaffolds can be precisely engineered to modulate PPIs, providing a roadmap for the development of next-generation peptide therapeutics targeting difficult-to-drug intracellular proteins like MCL-1.