Graspetide Biosynthesis
Reflecting work in the Link Group
The ribosomally synthesized and post-translationally modified peptide, RiPP, superfamily of natural products includes many examples of cyclic peptides with diverse macrocyclization chemistries. The graspetides, one family of macrocyclized RiPPs, harbor side chain–side chain ester or amide linkages. The Link Group at Princeton University recently reported the structure and biosynthesis of the graspetide pre-fuscimiditide, a 22-amino-acid (aa) peptide with two ester cross-links forming a stem–loop structure. These cross-links are introduced by a single graspetide synthetase, the ATP-grasp enzyme ThfB.

The Link Group
Now, published in JACS the Link Group shows that ThfB can also catalyze the formation of amide or thioester cross-links in prefuscimiditide, with thioester formation being especially efficient. They further show that upon proteolysis to reveal an N-terminal cysteine residue, the thioester-linked peptide rapidly and quantitatively rearranges via native chemical ligation into an isopeptide-bonded head-to-tail cyclic peptide. The solution structure of this rearranged peptide was determined by using 2D NMR spectroscopy experiments. This methodology offers a straightforward recombinant route to head-to-tail cyclic peptides.
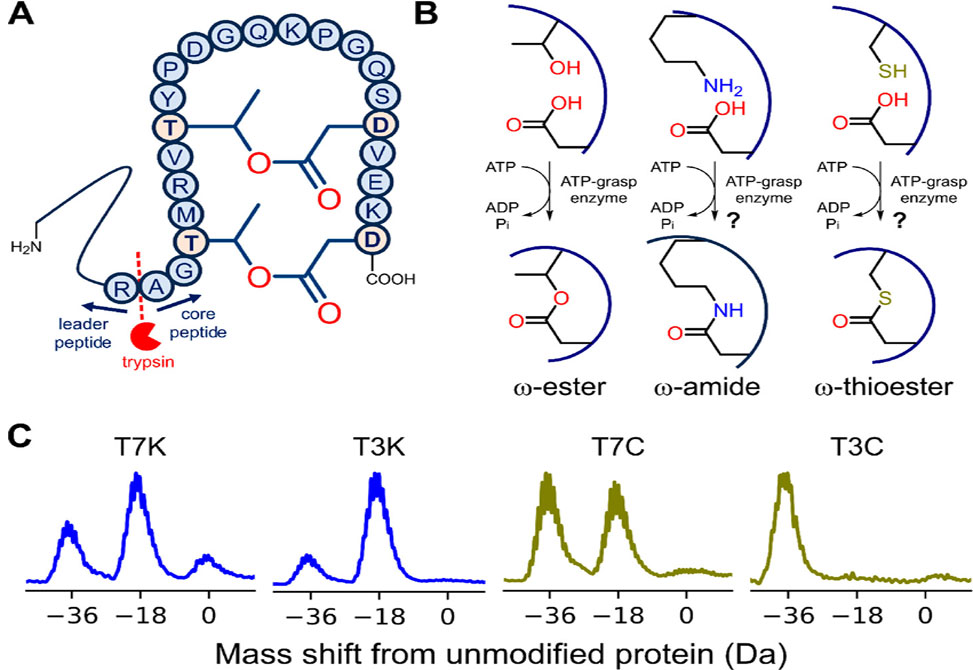
Formation of sidechain-to-sidechain linkages in pre-fuscimiditide. (A) Diagram showing the connectivity of ThfA with its core peptide modified by the ATP-grasp enzyme ThfB. Trypsin cleavage separates the leader peptide and the 22 aa core peptide, which harbors T3–D22 and T7–D18 ω-ester linkages. (B) Cross-links in ThfA catalyzed by ThfB. The ThfB enzyme natively installs ester cross-links between Thr and Asp, left; this study probes whether amide or thioester cross-links are also possible. (C) Deconvoluted mass spectra of ThfB-modified ThfA variants designed to harbor an ω-amide, blue, or ω-thioester linkage, green. 2-fold dehydration observed across the variants suggests the nonnative ω-amide or ω-thioester linkage formation.

