Guided Precision
Reflecting work in the Waser Group
Cysteine residues attract chemical attention. Their nucleophilic thiol groups react readily with electrophiles, making them popular handles for attaching drugs, fluorophores, and other payloads to proteins. Yet this reactivity creates a targeting problem: most proteins contain multiple cysteines, and standard reagents modify all accessible ones indiscriminately. A team led by Jerome Waser at the Ecole Polytechnique Fédérale de Lausanne, Switzerland, published in JACS, has now solved this selectivity challenge by combining hypervalent iodine reagents with peptide-based affinity guides, achieving single-cysteine precision on a protein bearing nine reactive sites.
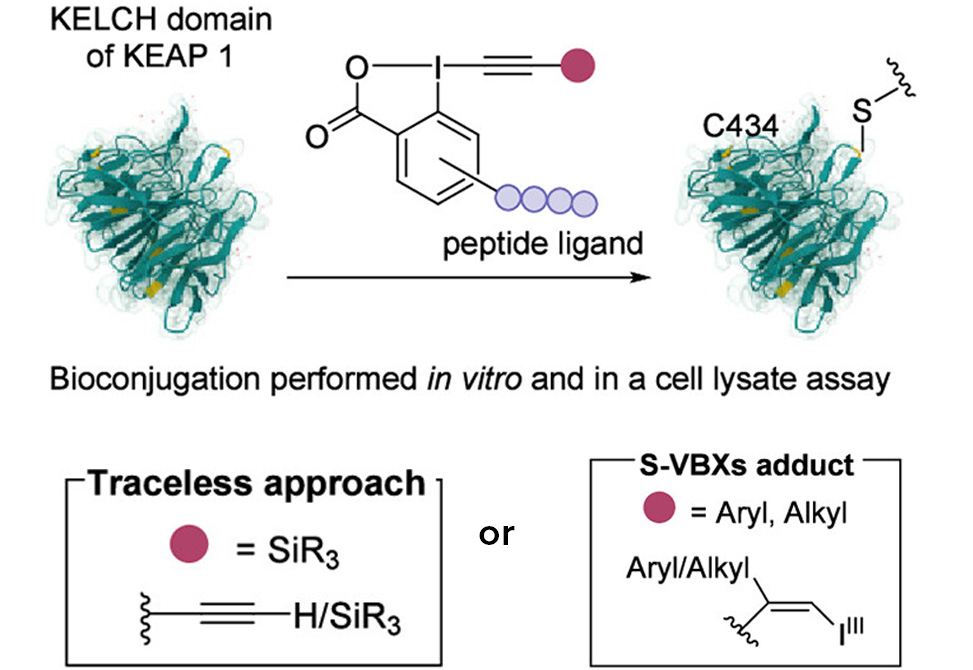
The target, the KELCH domain of KEAP1, plays a central role in cellular defense against oxidative stress. KEAP1 normally marks the transcription factor NRF2 for destruction, but when cells encounter oxidative damage, modifications to specific KEAP1 cysteines release NRF2 to activate protective genes. Among the nine free cysteines in KELCH, Cys434 sits near the pocket where NRF2 binds through a short ETGE peptide motif. The researchers recognized that peptides containing this motif could serve as molecular guides, delivering reactive cargo directly to Cys434 while ignoring the other eight cysteines scattered across the protein surface.
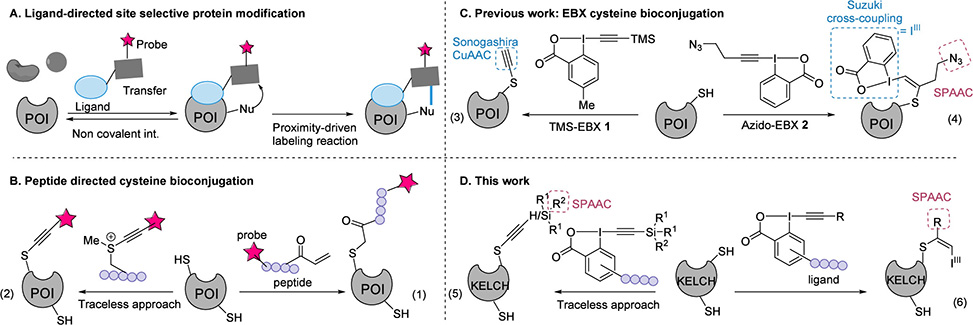
Scheme 1 Schematic Representation of Previous Methods and the Site-Selective Cysteine Bioconjugation with EBX Reagents Used in This Work. (A) Ligand-directed site-selective protein modification; (B) peptide-directed cysteine bioconjugation; (C) modification of cysteine with EBX reagents; and (D) this work, site-selective cysteine bioconjugation with EBX reagents.
The team designed new ethynylbenziodoxolone reagents bearing KEAP1-binding peptides attached to the aromatic core rather than the alkyne terminus. This geometry proved essential: when the peptide binds its target pocket, the hypervalent iodine warhead points directly at Cys434. The researchers synthesized reagents carrying silylated, arylated, and alkylated alkynes, then coupled them to peptide sequences on solid phase. Treatment of KELCH with the nine-residue ligand LDEETGEFL attached to a TIPS-EBX scaffold delivered 65% conversion to the alkynylated product. A mutant protein lacking Cys434 showed zero modification, confirming exquisite site selectivity. Scrambling the peptide sequence or mutating its key acidic residues eliminated labeling entirely, proving that affinity drives the reaction.
The chemistry operates in two distinct modes depending on the alkyne substituent. Silylated EBX reagents act as traceless delivery vehicles: they transfer only the alkyne to cysteine while releasing the peptide ligand. This preserves the native binding surface for subsequent biological studies. In contrast, aryl and alkyl EBX reagents form stable S-vinylbenziodoxolone adducts that retain the peptide attachment. These conjugates can be cleaved later using palladium-mediated reduction, offering temporal control over ligand release. Under one set of conditions the peptide departs cleanly; under slightly modified conditions the entire modification reverses, regenerating unmodified protein.
The researchers demonstrated practical utility through sequential functionalization. After labeling Cys434 with an azide-bearing EBX, they clicked on a fluorescein dye via strain-promoted azide-alkyne cycloaddition in a one-pot procedure. The approach tolerated complex biological mixtures remarkably well. In competition experiments with bovine serum albumin, which contains its own reactive cysteine, only KELCH underwent modification. Even in crude cell lysates containing thousands of proteins and abundant glutathione, the fluorescent signal localized specifically to KELCH.
This work establishes peptide-directed hypervalent iodine chemistry as a practical tool for single-site protein modification. The KEAP1-NRF2 axis represents an important therapeutic target, and the ability to install probes, drugs, or imaging agents at precisely Cys434 opens new avenues for studying and modulating this stress-response pathway. More broadly, the strategy should extend to any protein-protein interaction where a short peptide motif mediates binding, potentially bringing site-selective chemistry to targets that have resisted small-molecule approaches.
Publication Information
Author Information

Christine Marty earned her Ph.D. from the École Nationale Supérieure de Chimie de Montpellier, France. Ince 2022, she joined Jerome Waser’s group at EPFL in Switzerland and is now working primarily on the development of new hypervalent iodine reagents to perform site-selective modification on proteins.

