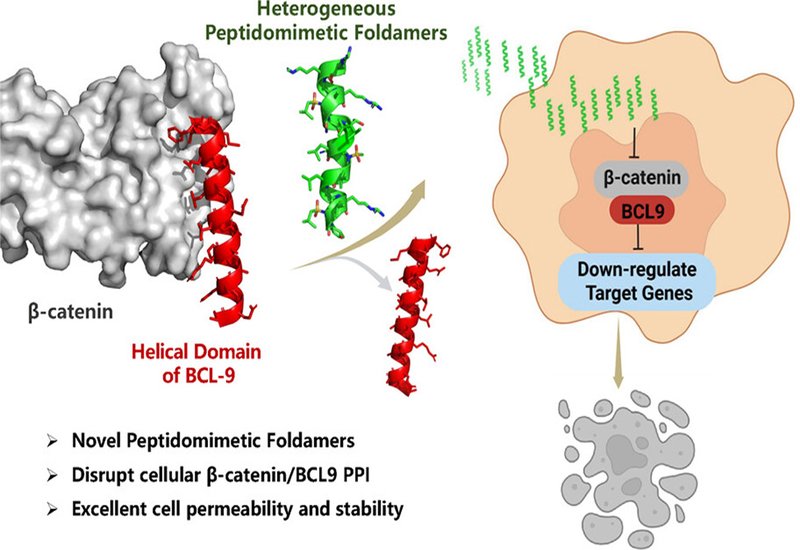
The Wnt/β-catenin signaling pathway governs embryonic development, stem cell maintenance, and tissue homeostasis. When aberrantly activated, it drives colorectal cancer and other malignancies. Inside the nucleus, β-catenin assembles with transcription factors and the coactivator B-cell lymphoma 9, BCL9, to switch on oncogenic genes. Disrupting the β-catenin/BCL9 protein-protein interaction, PPI, offers an attractive therapeutic strategy, but the interface is large, flat, and devoid of deep pockets, making it a difficult target for conventional small molecules.
Peptides can engage such surfaces through extended contact points, yet natural sequences suffer from poor proteolytic stability, limited cell permeability, and conformational flexibility in solution. Peptidomimetics, which incorporate nonnatural backbones, overcome many of these limitations. A team led by and Yan Shi, Jianfeng Cai, Jian Zhang, and Peng Sang, published in the Journal of the American Chemical Society, now reports a series of heterogeneous 1:1 α/sulfonyl-γ-AApeptides designed to mimic the helical domain of BCL9 and block its interaction with β-catenin.
The researchers drew on earlier crystallographic work showing that 1:1 α/sulfonyl-γ-AApeptides adopt right-handed helices with a pitch of 5.34 Å, closely matching the 5.4 Å pitch of a natural α-helix. This structural similarity enabled straightforward mimicry of the BCL9 HD2 domain, whose hotspot residues, Arg359, Leu363, Leu366, Ile369, and Leu373, nestle into a groove on β-catenin. The team positioned hydrophobic sulfonyl-isobutyl groups and amino acid side chains at corresponding helical positions, then iteratively optimized the sequence. Fluorescence polarization assays revealed that the most potent compound, peptide 13, bound β-catenin with a dissociation constant of 40 nM, roughly 25-fold tighter than the native BCL9 peptide.
Circular dichroism spectra confirmed that the lead compounds adopted well-defined right-handed helical conformations in solution. Confocal microscopy showed strong cellular uptake in Wnt-hyperactive colorectal cancer cell lines at concentrations as low as 1 μM, whereas the native BCL9 peptide exhibited negligible permeability. Biotinylated analogs pulled down β-catenin from cell lysates in a dose-dependent manner, and coimmunoprecipitation experiments demonstrated that the peptidomimetics reduced the amount of BCL9 associated with β-catenin inside cells. Quantitative PCR and RNA sequencing confirmed downregulation of Wnt target genes, including LEF1, AXIN2, and CD44. In proliferation assays, lead compounds inhibited Wnt-hyperactive cell lines with IC50 values in the low micromolar range while sparing nonmalignant cells at comparable concentrations. Serum stability tests revealed that the sulfonyl-γ-AApeptide backbone resisted degradation far better than the native peptide, which lost nearly 70 percent of its integrity within 24 hours.
By combining the target-engagement capacity of peptides with the stability and permeability of peptidomimetics, these right-handed foldamers offer a promising scaffold for disrupting intracellular PPIs. The approach may extend beyond β-catenin/BCL9 to other oncogenic interfaces that have long eluded small-molecule intervention.