Helical Hydrazides
Reflecting work in the Gellman Lab
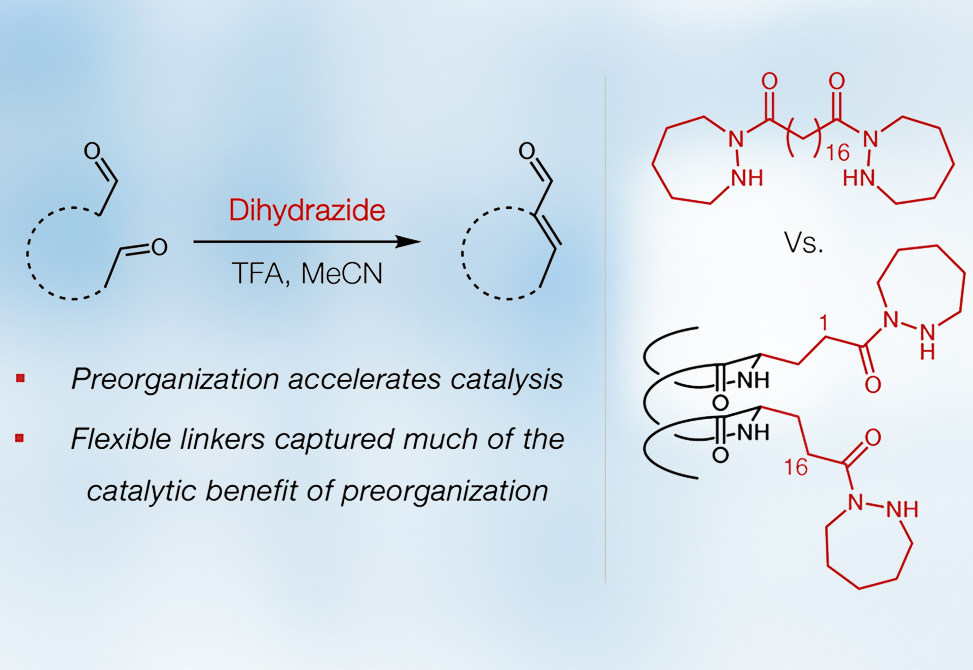
Enzymes are thought to accelerate reactions through precise positioning of reactive groups, substrate desolvation, tailored electrostatics, and coupling of structural dynamics to reaction coordinates. Efforts to develop synthetic catalysts have often attempted to mimic these features by organizing multiple reactive groups on molecular scaffolds. Previous work showed that a simple seven-membered ring hydrazide catalyzes aldol condensations of aldehydes. An apparent second-order kinetic dependence on the hydrazide was observed, suggesting a bifunctional mechanism involving simultaneous iminium and enamine activation of aldehyde substrates by hydrazide units. Flexible dihydrazides composed of two hydrazide units connected with a 10-methylene linker provided enhanced rates relative to a monohydrazide, underscoring the value of covalently linking two hydrazide groups for cooperative catalysis.
Seeking to understand the relative kinetic benefits of reactive unit connection and preorganization, researchers from the Gellman Lab at the University of Wisconsin–Madison investigated α/β-peptide foldamers containing a preorganized hydrazide diad. Their report was published in the Journal of the American Chemical Society. The α/β-peptide scaffold, featuring a 1:2 α:β residue pattern and helical conformation, was hypothesized to align hydrazide units in the i,i+3 spacing along one side of the helix. This design was expected to restrict conformational freedom and favor cooperative bifunctional catalysis by preorganizing the hydrazide units next to one another.
An α/β-peptide heptamer catalyst, containing two glutamic acid residues connected to hydrazide groups in the i,i+3 spacing, exhibited significant activity in the homoaldol condensation of hydrocinnamaldehyde, achieving nearly quantitative product yield after 1 h and a νREL of 89 compared to a simple monohydrazide. Variants containing hydrazide-displaying residues with shorter or longer side chains showed reduced activity, indicating there was an optimal spacing between the reactive hydrazide groups. Comparisons with flexible dihydrazides revealed that the α/β-peptide heptamer dihydrazide conferred a 2.5- to 3.5-fold enhancement in initial reaction rate, a modest improvement relative to expectations based on the conformational preorganization offered by the peptides. Structural analysis yielded the first crystal structures of catalytically active α/β-peptides that showed consistent helical backbones but variable side chain orientations, highlighting that the α/β-peptide heptamer catalysts could closely arrange the hydrazide units while still allowing for appreciable flexibility.
This work demonstrates that α/β-peptide foldamer catalysts can preorganize a catalytic diad and provide measurable rate enhancements over catalysts with simple flexible linkers. However, the modest magnitude of improvement suggests that simple covalent connection between reactive groups delivers much of the available benefit, and that enzyme-like rate acceleration likely requires additional contributions such as substrate desolvation or multipoint interactions. These findings provide a foundation for future exploration of foldamer-based catalytic scaffolds.
Author Information

Philip P. Lampkin is a graduate student in the Department of Chemistry at the University of Wisconsin–Madison. His work, conducted under the supervision of Professor Sam Gellman, examines the properties of bifunctional catalysts with flexible and foldamer molecular scaffolds. Outside his research efforts involving flexible and foldamer catalysts, Philip developed an open source photoreactor platform and designed an advanced organic chemistry lab curriculum. In January 2026, he will begin a postdoctoral fellowship in the lab of Professor Matt Sigman at the University of Utah.

