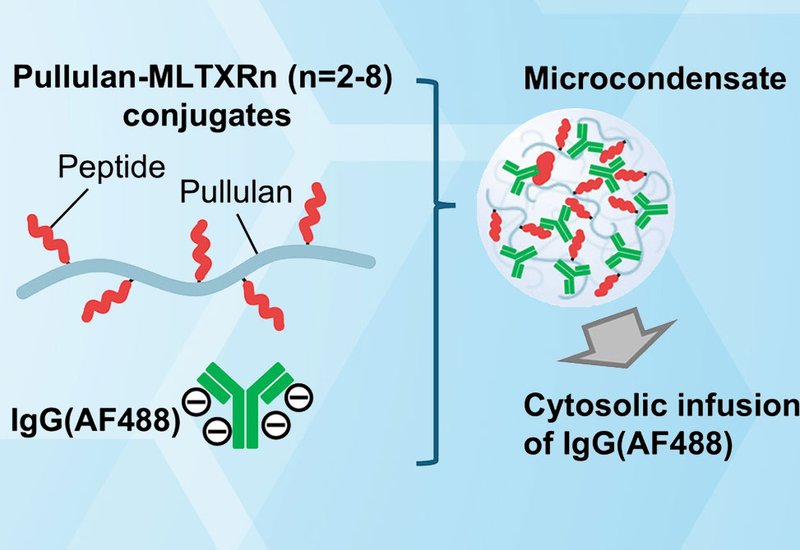
The delivery of biomacromolecules directly into the cytosol remains a central challenge in therapeutic peptide science. Coacervate-based systems offer a promising platform, yet many are hampered by instability in physiological conditions and diminished performance in serum-containing media. In this study, published in Bioconjugate Chemistry, by researchers from the Futaki Group at Kyoto University, present a refined, polysaccharide-based microcondensate system that significantly enhances the intracellular delivery efficiency of immunoglobulin G, IgG, antibodies while reducing the required dosage.
Building on prior work utilizing pullulan conjugates with membrane-permeabilizing peptides, notably L17E and M-lycotoxin, the investigators synthesized a suite of new delivery vectors by conjugating pullulan to M-lycotoxin peptides bearing 2–8 terminal arginine residues, PL-MLTXRn. These arginine modifications were designed to strengthen membrane interaction, coacervate formation, and cellular uptake.
Among the variants, PL-MLTXR4, bearing four arginines, demonstrated optimal performance. Compared to the unmodified PL-MLTX, the PL-MLTXR4 system achieved cytosolic IgG delivery to ~70% of HeLa cells at only 10 μg/mL IgG, AF488, and ~85% at 20 μg/mL — representing a threefold reduction in required antibody concentration for comparable efficacy. The delivery process was rapid, with cytosolic dispersion observed within 1 minute of microcondensate-cell contact. Notably, PL-MLTXR4 microcondensates maintained structural stability and activity in serum-containing media, an essential criterion for in vivo applications.
Liposome leakage assays confirmed enhanced membrane-disrupting capabilities for PL-MLTXR4 compared to its unmodified counterpart. Confocal microscopy demonstrated that the microcondensates formed discrete, stable particles capable of penetrating various cell lines, including A549, HT1080, and MIA PaCa-2, with significantly higher delivery efficiencies than PL-MLTX alone. Importantly, delivered IgG retained biological function, as shown by anti-nuclear pore complex antibodies targeting the nuclear envelope post-delivery.
This work validates the PL-MLTXR4-based microcondensate system as a robust, efficient, and scalable method for intracellular IgG delivery, offering significant advantages in terms of dose economy, speed, and bioactivity preservation. These findings support the future development of arginine-tuned coacervate platforms for therapeutic protein delivery and intracellular targeting applications.