Lasso Peptides
Reflecting work in the Swanson lab
Lasso peptides make up a class of natural products characterized by a threaded structure. Given their small size and stability, chemical synthesis would offer tremendous potential for the development of novel therapeutics. However, the accessibility of the pre-folded lasso architecture has limited this advance.
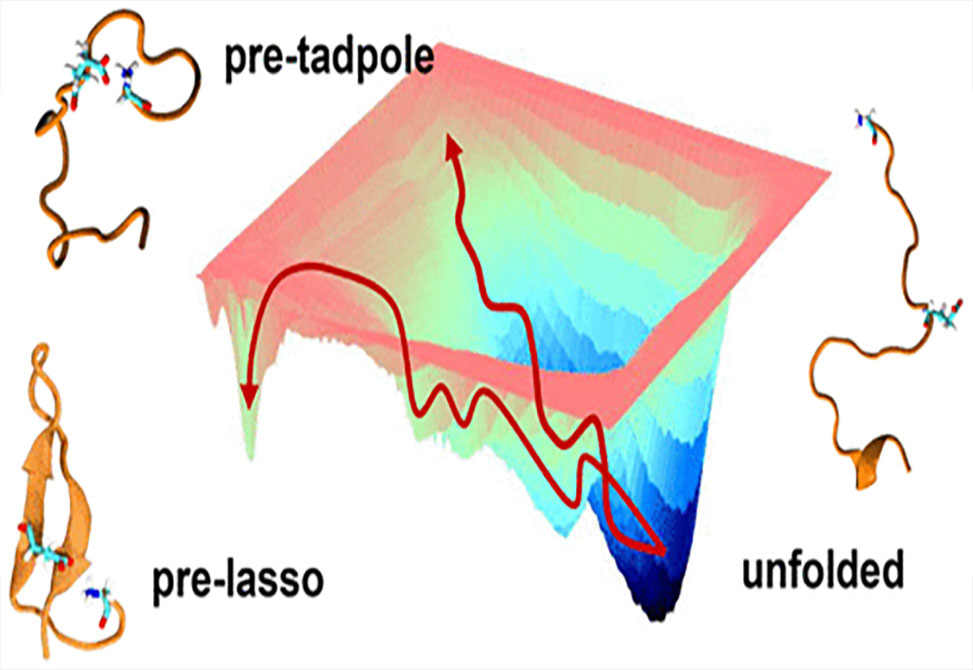
To better understand the folding process de novo, simulations are used in work published in JACS, by the Swanson Group to characterize the folding propensity of microcin J25, MccJ25, a lasso peptide known for its antimicrobial properties. New algorithms were developed to unambiguously distinguish threaded from nonthreaded precursors and determine handedness, a key feature in natural lasso peptides.

Group members found that MccJ25 indeed forms right-handed pre-lassos, in contrast to past predictions but consistent with all natural lasso peptides. Additionally, the native pre-lasso structure is shown to be metastable prior to ring formation but to readily transition to entropically favored unfolded and nonthreaded structures, suggesting that de novo lasso folding is rare. However, by altering the ring forming residues and appending thiol and thioester functionalities, group members were able to increase the stability of pre-lasso conformations. Furthermore, conditions leading to protonation of a histidine imidazole side chain further stabilize the modified pre-lasso ensemble.
This work highlights the use of computational methods to characterize lasso folding and demonstrates that de novo access to lasso structures can be facilitated by optimizing sequence, unnatural modifications, and reaction conditions like pH.