Lipopeptidomimetics Tool
Reflecting work in the Mapp Lab
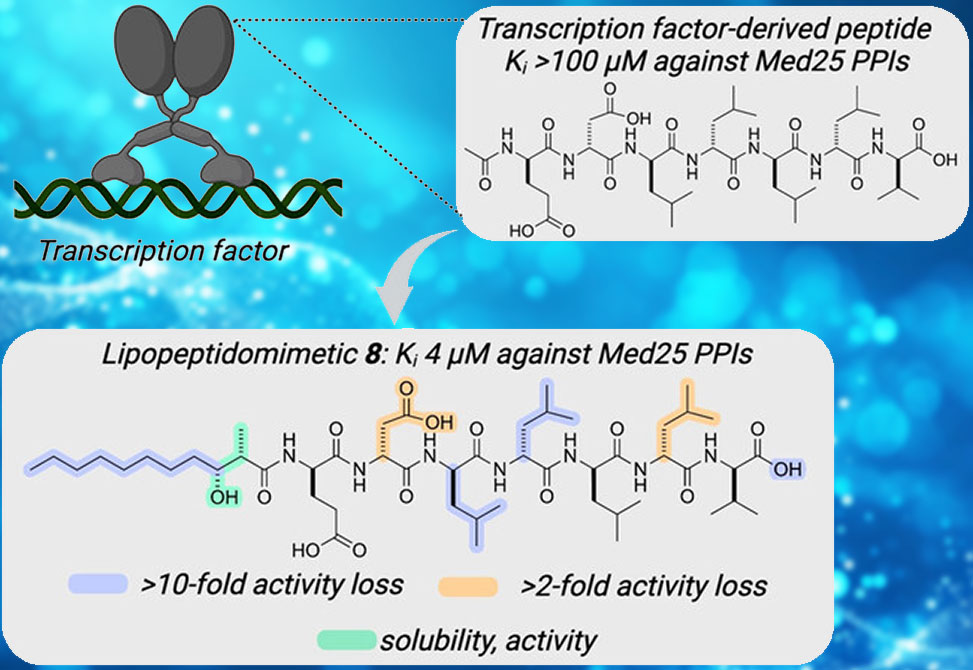
Short amphipathic peptides are capable of binding to transcriptional coactivators, often targeting the same binding surfaces as native transcriptional activation domains. However, they do so with modest affinity and generally poor selectivity, limiting their utility as synthetic modulators.
A collaborative study led by Professor Anna Mapp, involving researchers from the Life Sciences Institute, Program in Chemical Biology, and the Departments of Chemistry, Internal Medicine - Hematology/Oncology, and Pathology at the University of Michigan, published in Angewandte Chemie Intl. Ed., demonstrates that adding a medium-chain, branched fatty acid to the N-terminus of the lipopeptidomimetic LPPM-8 increases its affinity for the coactivator Med25 by over 20-fold, from Ki > 100μM to 4μM, making it an effective inhibitor of Med25 protein–protein interactions.
The lipid structure, the peptide sequence, and the C-terminal functionalization of the lipopeptidomimetic each influence the structural propensity of LPPM-8 and its effectiveness as an inhibitor. LPPM-8 engages Med25 through interaction with the H2 face of its activator interaction domain and in doing so stabilizes full-length protein in the cellular proteome. Further, genes regulated by Med25-activator PPIs are inhibited in a cell model of triple-negative breast cancer.
Thus, LPPM-8 is a useful tool for studying Med25 and mediator complex biology and the results indicate that lipopeptidomimetics may be a robust source of inhibitors for activator-coactivator complexes.