Masked Sugars
Reflecting work in the Dong Group
Chemical synthesis provides atomic-level control over glycoprotein structure, yet hydrophobic sequences often aggregate before chemists can purify or ligate them. This problem may intensify for some sequences bearing only a single N-acetylglucosamine, GlcNAc, residue, the minimal glycan commonly used as a starting point for building complex N-glycans. Existing strategies for difficult sequences, such as removable backbone modifications or solubilizing tags, were not designed with carbohydrate chemistry in mind, and many employ harsh conditions incompatible with glycosidic bonds.
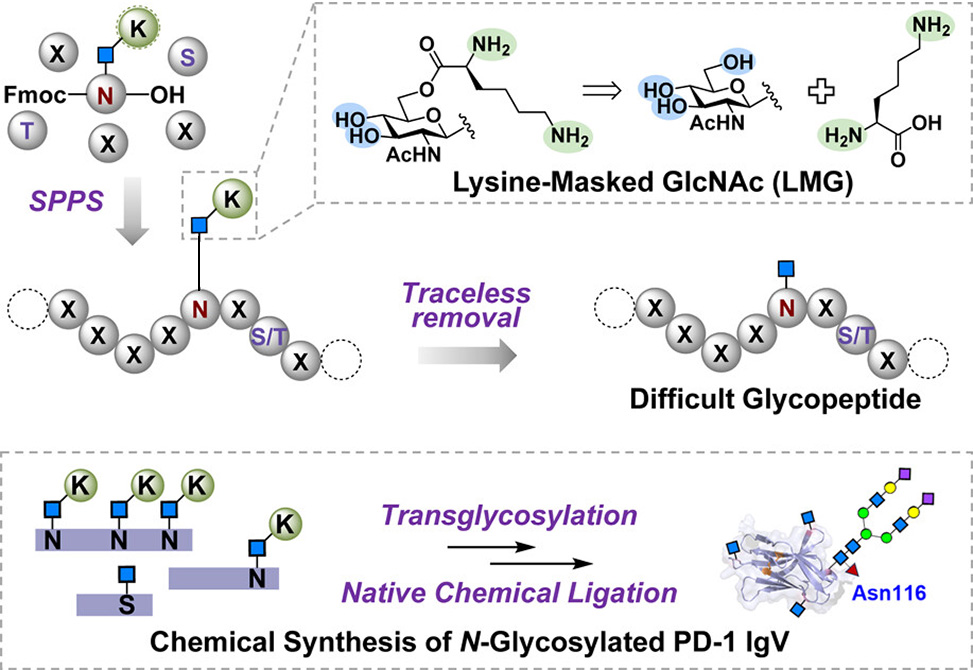
A team led by Suwei Dong at Peking University, published in the Journal of the American Chemical Society, introduces a lysine-masked GlcNAc, LMG, strategy that esterifies a protected lysine onto the 6-hydroxyl group of GlcNAc linked to asparagine or serine. The positively charged lysine tail dramatically improves solubility while remaining stable throughout standard Fmoc solid-phase peptide synthesis. Mild neutral aqueous conditions, identical to those used for native chemical ligation, then hydrolyze the ester and regenerate the native sugar without additional reagents.

Figure 1. Schematic depiction of the “cassette” strategy and LMG strategy for accessing GlcNAcylated peptides.
The researchers validated LMG on notoriously aggregation-prone sequences from TMEM106B, a lysosomal membrane protein implicated in neurodegeneration, and from the cancer-associated receptor MST1R. Traditional synthesis yielded insoluble gels; LMG-modified peptides dissolved readily and purified in high yield. Extending the approach to O-GlcNAc, the team prepared a serine building block and used it to access an otherwise intractable fragment of interleukin-2. LMG-bearing intermediates also proved compatible with chemoenzymatic glycan elaboration: α1,6-fucosylation and endoglycosidase-catalyzed transfer of complex sialooligosaccharides proceeded efficiently, with the lysine tag hydrolyzing in situ and releasing reactive substrate below its aggregation threshold.
The strategy culminated in a three-segment assembly of the PD-1 IgV domain bearing four site-specific N-glycans. Surface plasmon resonance measurements revealed that GlcNAc alone at Asn116 permits rapid association with PD-L1 but also fast dissociation, whereas a bulky core-fucosylated dodecasaccharide slows both processes. This trade-off between kinetic accessibility and complex stability offers fresh mechanistic insight into how glycan composition tunes immune checkpoint signaling. In sequences otherwise prone to aggregation, LMG transforms the minimally glycosylated motif into a solubilizing handle, expanding the synthetic toolkit for probing glycan function in disease-relevant proteins.