Metallo-Azapeptides
Reflecting work in the Proulx Lab
Published in the Journal of the American Chemical Society, researchers in the Proulx group at the North Carolina State University at Raleigh, present the first approach to controlled metal chelation of peptide backbones, where the anchoring site is an aza-amino acid nitrogen and the directionality of chelation events is dictated by the acidity of neighboring NHs.
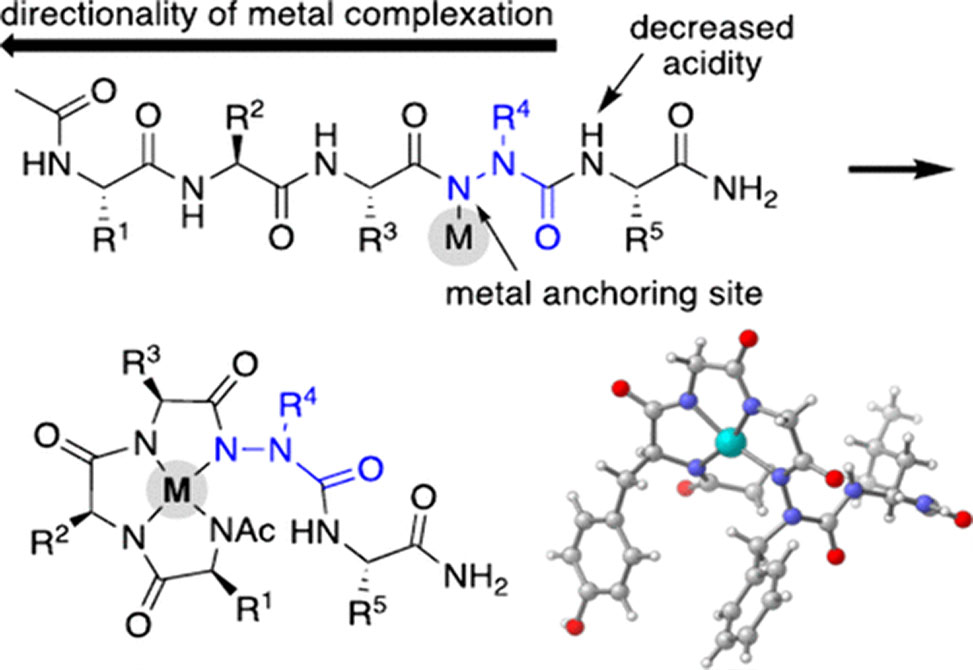
Selective backbone chelation precludes the need for metal-binding side chains and/or free N- or C-termini in peptides. The group members show that the presence and location of an aza-amino acid impact complex formation and report the first X-ray crystal structures of azapeptides bound to palladium and nickel. Evidence of atropisomerism in metallo-azapeptides is also presented.