Mirror-Image mRNA
Reflecting work in the Payne Lab
Chemokines are small proteins involved in recruiting leukocytes to sites of inflammation via interactions with specific cell surface receptors. CCL22 is a chemokine known to play a critical role in inflammatory diseases such as atopic dermatitis and asthma - inhibition of this chemokine therefore represents an attractive therapeutic strategy.
Published in the Journal of the American Chemical Society, researchers in the Payne Group at the University of Sydney, describe the discovery of cyclic D-sulfopeptide inhibitors of CCL22 identified through mirror-image mRNA display with genetic reprogramming.

The Payne Group
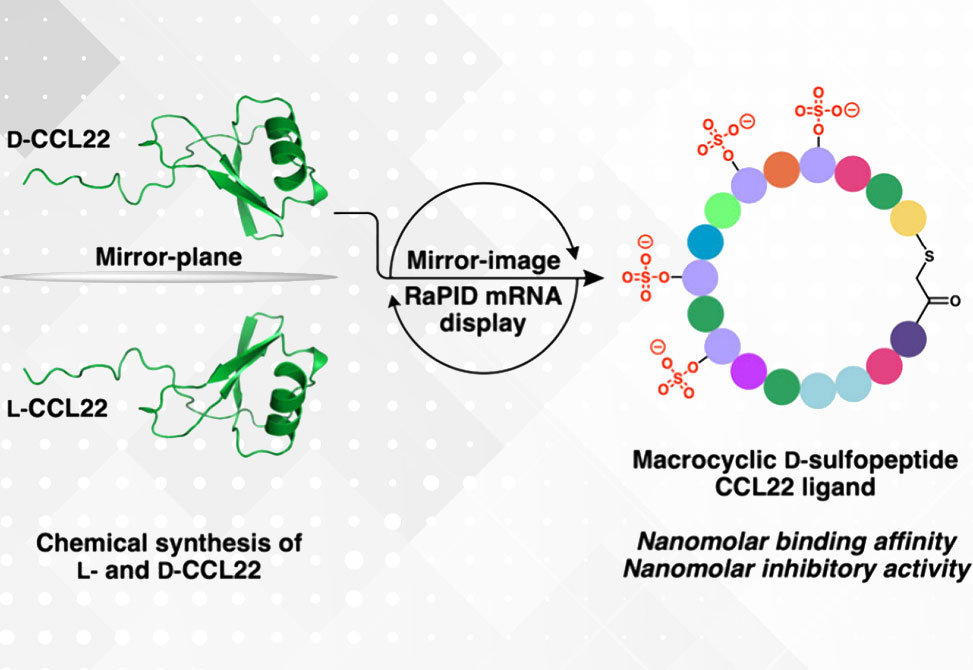
Chemical synthesis of mirror-image D-CCL22 enabled screening of a cyclic peptide library comprised of all L-amino acids, with reprogramming of L-sulfotyrosine to mimic the presence of this post-translational modification on native chemokine receptors. Enriched macrocyclic peptides were prepared in their mirror-image D-form and assessed for binding against native L-CCL22.
The most potent ligand, a plasma-stable D-cyclic peptide bearing four D-sulfotyrosine residues, exhibited nanomolar affinity for CCL22, high selectivity over other chemokines, and nanomolar inhibition of CCL22 signaling through CCR4.
This work highlights the vast potential of mirror-image mRNA display technology for discovering proteolytically stable D-peptide inhibitors of protein–protein interactions relevant across a range of therapeutic indications.