This comprehensive review from the labs of David Craik and Lara Malins at Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science, at the University of Queensland, published in Biochemistry, highlights the powerful therapeutic potential of cyclic and disulfide-rich peptides as robust scaffolds for intracellular drug delivery. Focusing on naturally occurring and engineered peptides from plants, animals, and human proteins, the article details their structural features, cell-penetrating mechanisms, and capacity to deliver bioactive cargos to cytosolic targets.
Particular attention is given to cyclotides, especially the trypsin-inhibitor MCoTI-II and Möbius-type kalata B1, which exhibit remarkable proteolytic stability and selective internalization via endocytic or lipid-targeting pathways. The authors show how MCoTI-II can be modified to deliver peptide inhibitors that disrupt protein–protein interactions in cancer and stress-response pathways. Kalata B1, despite its more limited amenability to modification, displays superior uptake via specific interactions with phosphatidylethanolamine-rich membranes.
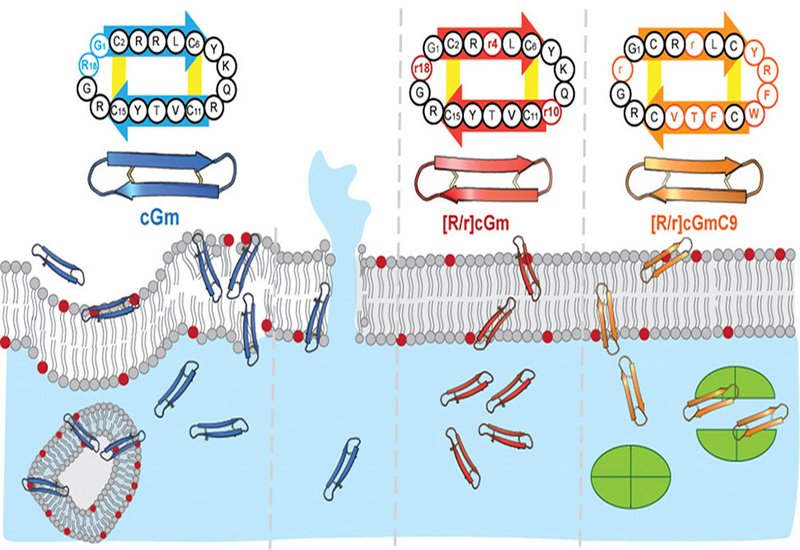
The review also explores β-hairpin peptides like gomesin and tachyplesin I, which combine strong membrane affinity with selective toxicity and can be cyclized to enhance cellular uptake and target delivery. The team presents examples where these scaffolds are engineered to disrupt cancer metabolism, kill intracellular bacteria, or inhibit challenging cytosolic targets such as LDH5 and p53–MDM2/MDMX complexes.
In addition, the authors introduce PDIP—a synthetic, α-helical peptide inspired by human platelet factor 4—that selectively penetrates malaria-infected red blood cells and cancer cells. PDIP and its analogs are used to deliver both peptides and small-molecule drugs through cleavable and non-cleavable linkers, demonstrating modular design and broad therapeutic scope.
Finally, the article highlights recent advances in de novo peptide design, showing that compact, internally hydrogen-bonded macrocycles can achieve cell permeability and even oral bioavailability. These findings underscore the growing convergence of nature-inspired scaffolds with AI-driven peptide engineering, paving the way for next-generation peptide therapeutics targeting previously "undruggable" intracellular pathways.