Non Classical Crystallization
Reflecting work in the Kellermeier Lab
In a recent study, published in Advanced Functional Materials, researchers in the Kellermeier Lab at BASF, have used phage display screening to identify peptide motifs with strong and selective affinity to bind to the surfaces of gypsum, an inorganic mineral of high abundance in geo(bio)chemical environments and large-scale industrial use for construction purposes. The goal of the present work was to investigate the influence of such engineered peptides on the formation of gypsum crystals under different conditions.
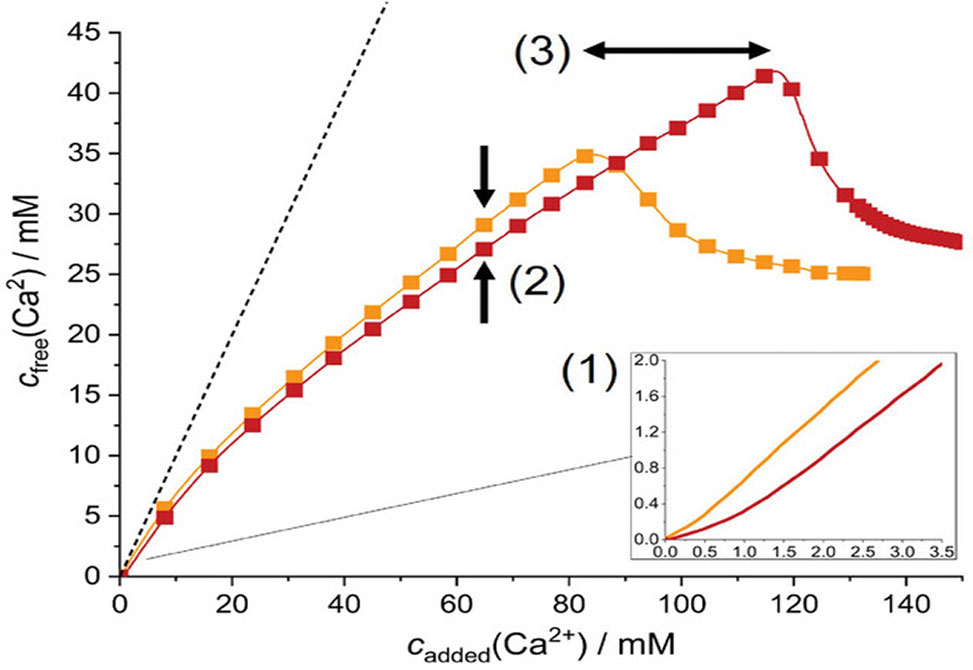
To establish a holistic picture, the previously discovered multi-stage mineralization pathway of gypsum was scrutinized in detail by a combination of complementary characterization techniques, including potentiometric titration and cryogenic transmission electron microscopy. The results provide clear evidence that the added peptide interferes with practically all precursor and intermediate species on the way from dissolved ions to solid crystals. Key to this multifunctional behavior is the self-assembly of the peptides to filamentous colloidal aggregates in the presence of calcium ions, which depends on peptide molecular weight and related changes in secondary structure formation.
Overall, the findings made in this work highlight the potential of biotechnological selection techniques for the design of advanced concepts for crystallization control, including supramolecular peptide self-assembly as a principle known from natural biomineralization and of utmost value for bioinspired material science.