Peptide Anti Obesity 2.0
Reflecting recent work in the Craik, Gachin, and Chan Labs
Obesity is a leading contributor to global disease burden and a primary risk factor for type 2 diabetes, yet current treatments often lack specificity and long-term efficacy. In a groundbreaking collaborative study, published in the Journal of the American Chemical Society, researchers from the Craik, Chan, and Gachon Groups at the University of Queensland, report the development of a next-generation antiobesity peptide therapeutic—PTP-r—designed to target white adipose tissue, WAT, by homing in on the mitochondrial prohibitin, PHB, protein.
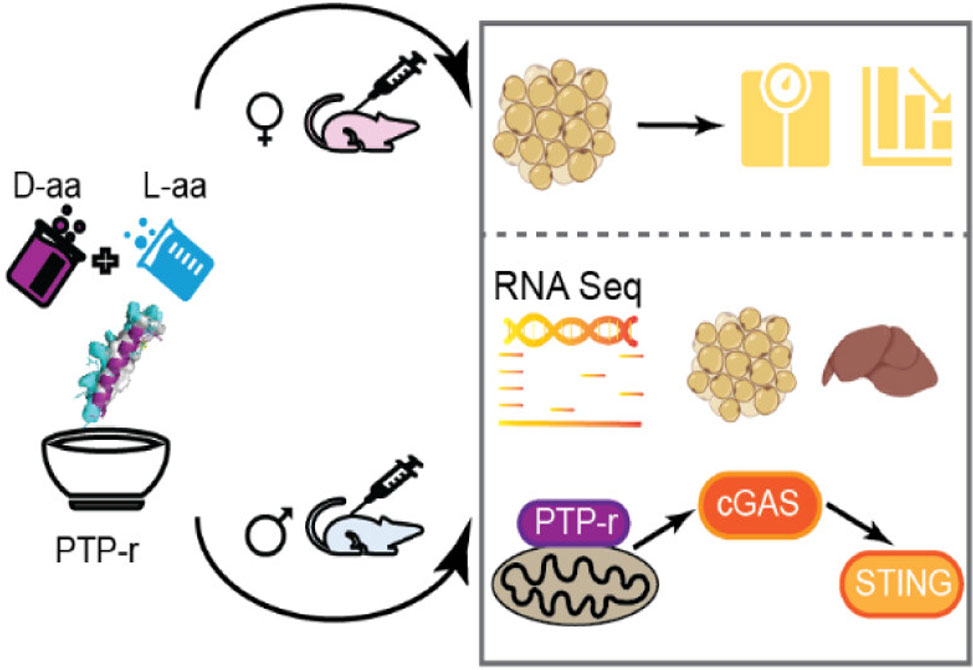
Derived from the chimeric peptide prohibitin-TP01, PTP, the newly engineered PTP-r incorporates D-arginine substitutions into the proapoptotic KLAK motif to enhance peptide stability, mitochondrial localization, and lipid-disruptive activity. The result is a potent compound that induces mitochondrial uncoupling and drives significant weight loss in high-fat diet-induced obese mice, all while exhibiting excellent safety profiles in preclinical models.
PTP-r binds preferentially to phosphatidylserine, PS-rich, membranes—abundant in WAT and mitochondrial inner membranes—showing selective internalization into adipocytes and effective disruption of mitochondrial membrane potential. Structure–activity relationship studies confirmed that D-amino acid incorporation prolongs peptide stability in serum and enhances helical structure integrity upon membrane engagement.
Treatment with PTP-r not only reduced lipid accumulation in mature 3T3-L1 adipocytes but also induced sex-specific metabolic changes. RNA-sequencing revealed upregulation of browning-associated genes, for example, Ucp1, Ppargc1a, Cidea, in female WAT, along with activation of mitochondrial stress signaling via the cGAS-STING pathway, particularly in male liver tissue. This dual action points to a nuanced, sex-dependent mechanism involving mitochondrial remodeling and immune signaling.
Subcutaneous administration of PTP-r led to rapid and sustained body weight reduction in obese mice without changes in food intake or liver toxicity. Treated animals exhibited smaller adipocytes and reduced WAT mass, supporting the peptide’s ability to mitigate fat expansion while preserving metabolic tissue health.
This work highlights PTP-r as a promising mitochondria-targeted peptide therapeutic capable of modulating adipocyte biology and systemic metabolism. Through rational design and biochemical fine-tuning, the study opens new avenues for safe, peptide-based interventions in obesity and metabolic syndrome, with the potential for precision application across sex-specific physiological contexts.