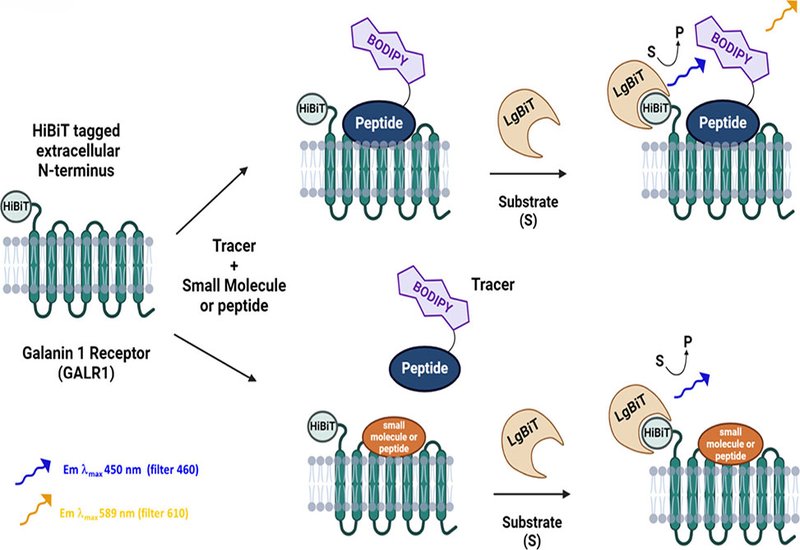
Galanin, a neuroendocrine peptide widely expressed in the central and peripheral nervous systems, regulates diverse physiological functions such as energy balance, mood, pain, and neuroendocrine signaling. These functions are mediated via three G protein-coupled receptor, GPCR, subtypes—GALR1, GALR2, and GALR3. Traditional methods for characterizing ligand-receptor interactions, such as radioligand binding assays, have proven limited by safety constraints, low throughput, and an artificial, membrane-isolated context. In a landmark study, published in ACS Chemical Biology, researchers from the Samarjit Patnaik group at the National Institutes of Health, report the development of a live-cell NanoBRET, Bioluminescence Resonance Energy Transfer, assay targeting GALR1, utilizing novel fluorescent peptide tracers derived from galanin, Gal(1–15), and M40 peptides.
The NanoBRET assay centers on a HiBiT-tagged GALR1 receptor expressed on HEK293 cells. Upon complementation with LgBiT, the donor luciferase generates bioluminescence on addition of furimazine substrate. When a fluorescently labeled ligand tracer binds in close proximity, <10 nm, resonance energy transfer yields a BRET signal. This system allows ratiometric quantification of ligand-receptor interactions in real time within intact cellular environments.
To construct compatible tracers, group members synthesized six peptide-fluorophore conjugates by attaching BODIPY-based NanoBRET 590 dyes to either native lysine residues or synthetic C-terminal lysines. These were derived from full-length galanin, 30 aa, Gal(1–15), 15aa, and M40, 20aa, each modified with two linker lengths to optimize energy transfer geometry. Tracers were rigorously characterized using LC/MS, radioligand competition assays, and multiple functional cellular readouts.
All six tracers demonstrated agonist activity in a β-arrestin recruitment assay, with EC50 values ranging from 6–33 nM. M40-derived tracers exhibited the highest potency, while Gal(1–15)-derived tracers showed moderate affinity. Binding saturation assays revealed a clear length-dependent decrease in BRET signal intensity—shorter peptides, Gal(1–15), yielded stronger signals, likely due to favorable donor–acceptor spatial orientation.
NanoBRET-derived equilibrium dissociation constants, K_D, ranged from ~10 to ~190 nM, closely aligned with kinetic values derived from real-time association/dissociation curves. M40 tracers had the strongest binding affinity, K_D ~10–18 nM, and tracer residence times ranged from 11 to 46 minutes, underscoring the assay's utility in kinetic profiling.
To validate tracer specificity, displacement assays were conducted with a panel of ten unmodified galanin-derived peptides. Affinities, K_i, spanned four orders of magnitude, with peptides like M15, M35, and galanin showing nanomolar potency, while truncated or modified sequences, for example, Gal(2–11), Gal(DMG,2–15), showed minimal or no binding. Notably, the NanoBRET assay reliably ranked ligand affinities consistent with historical radioligand data, but with expected right-shifts due to live-cell context and possible serum peptide sequestration.
Tracers also bound effectively to GALR2, though GALR3 saturation was not observed—likely due to poor receptor surface expression. Correlation analyses between BRET binding data and β-arrestin EC50 values confirmed strong alignment between binding and functional activation across peptide variants.
A complementary HiBiT-GALR1 internalization assay provided additional functional validation. Decreased luminescence upon ligand-induced endocytosis indicated receptor activation. Potent ligands such as M15 and M35 induced robust internalization, while others, for example, Gal(1–15), M617, showed moderate effects. Interestingly, M40 acted as a partial agonist in this assay—contrasting with its full agonist behavior in β-arrestin recruitment and its classical designation as an antagonist—highlighting potential functional selectivity.
Tracers were further benchmarked in radioligand displacement assays using [3H]galanin. Results showed strong concordance with NanoBRET-derived affinities, R2 = 0.845. However, the authors argue convincingly for the superiority of NanoBRET in live-cell pharmacology: unlike radioligand assays, NanoBRET preserves native receptor architecture, G-protein and ion allosteric modulators, and membrane lipid context. This enables more physiologically relevant insights into ligand behavior, especially for peptide GPCRs like GALR1.
This study establishes a robust, scalable NanoBRET assay system for monitoring galanin–GALR1 interactions in live cells. The six engineered peptide-based tracers provide tools for quantifying binding affinities, kinetics, receptor internalization, and functional activity with high precision and biological relevance. These results underscore the broader applicability of peptide-based NanoBRET tracers in GPCR pharmacology and reinforce the central role of live-cell assay systems in the rational design of neuropeptide therapeutics.