Peptide Gels
Reflecting work in the Schneider Lab
Researchers in the Schneider lab, published in Angewandte Chemie, Intl. Ed., discuss how peptide nucleic acids, PNAs, are employed in the design of a participatory duplex PNA-peptide crosslinking agent. Biophysical and mechanical studies show that crosslinkers present during peptide assembly leading to hydrogelation participate in the formation of fibrils while simultaneously installing crosslinks into the higher-order network that constitutes the peptide gel.
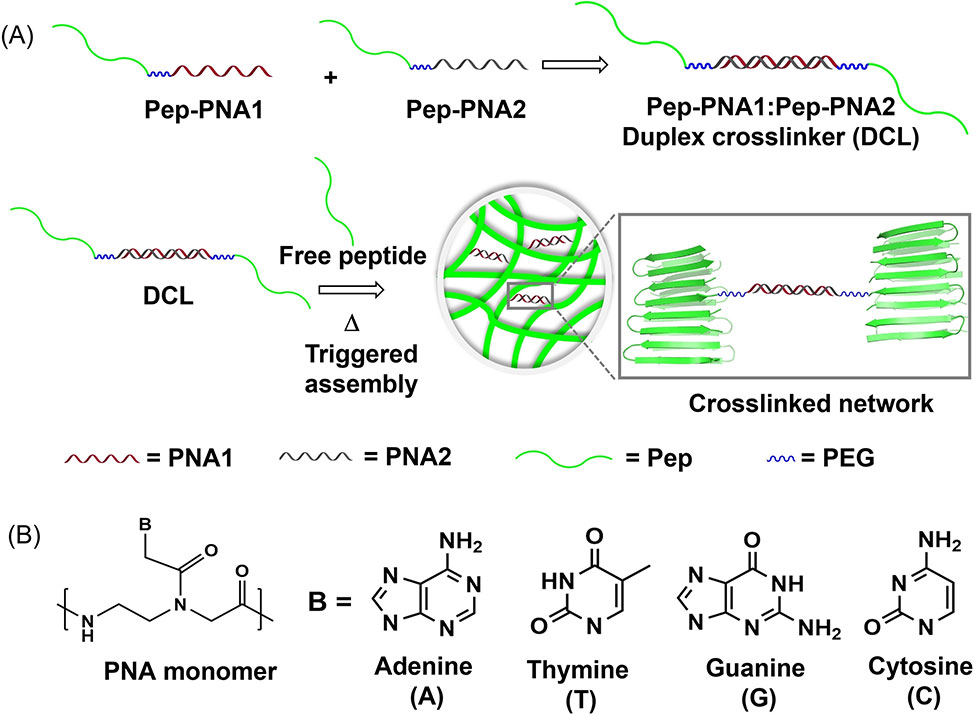
(A) PNA duplex crosslinker, DCL, formed by the hybridization of individual PNA-peptide monomers. When present during peptide assembly, the DCL participates in fibril formation while simultaneously crosslinking the evolving hydrogel network. (B) Structure of PNA monomer and nucleobases.
The addition of 2mol% crosslinker into the assembling system results in a ~100% increase in mechanical stiffness without affecting the rate of peptide assembly or the local morphology of fibrils within the gel network. Stiffness enhancement is realized by only affecting change in the elastic component of the viscoelastic gel. A synthesis of the PNA-peptide duplex crosslinkers is provided that allows facile variation in peptide composition and addresses the notorious hydrophobic content of PNAs.
This crosslinking system represents a new tool for modulating the mechanical properties of peptide-based hydrogels.
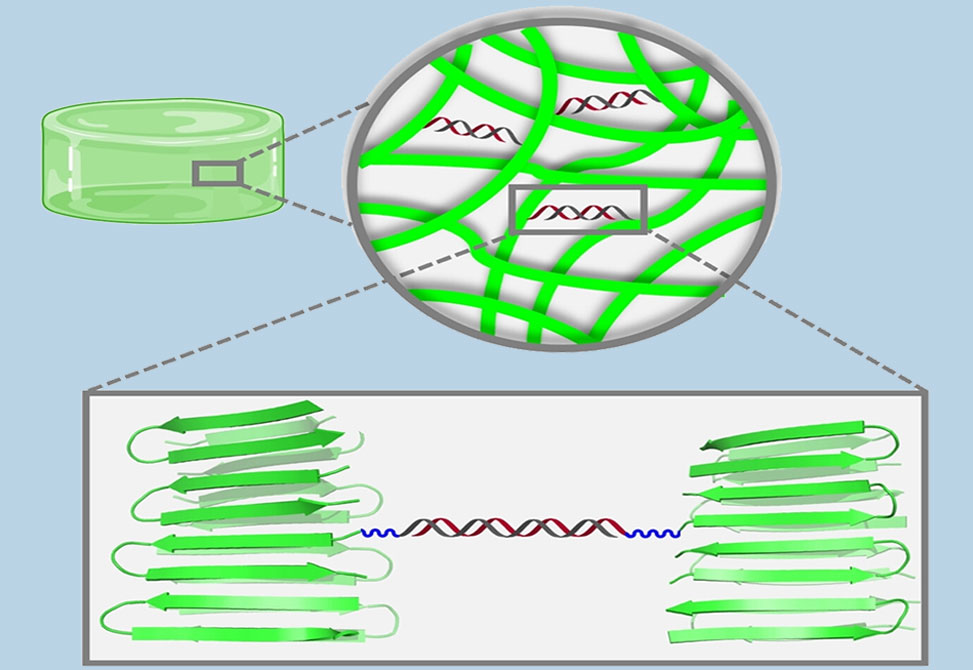
The development of crosslinking agents to modulate the mechanical properties of peptide-based gels increases their scope of utility. We report the design, synthesis and utilization of a peptide nucleic acid duplex crosslinker,DCL, that takes part in peptide assembly leading to gel formation while simultaneously installing non-covalent crosslinks into the higher-order fibrillar network. DCL represents a new modality in the crosslinker arsenal.

